Baekeland-Manasse-Lederer reaction
The Baekeland-Manasse-Lederer reaction is a name reaction in organic chemistry in which phenol reacts with an aldehyde to form a phenolic resin . The reaction was discovered in 1872 by the German chemist Johann Friedrich Wilhelm Adolf (since 1885 Ritter von) Baeyer (* October 31, 1835 - August 20, 1917), in which he synthesized a sticky substance with his "Bakelizer" apparatus, which he synthesized "Schmiere" called. The current reaction was researched and published independently by Otto Manasse and Leonhard Lederer in 1894. Today the resulting product is known as shellac substitute or bakelite .
Overview reaction
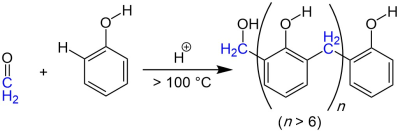
In the Baekeland-Manasse-Lederer reaction, phenol and formaldehyde react with one another in a chain reaction.
Reaction mechanism
In the proposed reaction mechanism, a formaldehyde molecule is first protonated and then attacks the aromatic ring of the phenol in the form of an electrophilic aromatic substitution . At over 100 ° C, dehydration and a reaction with another phenol molecule follow . Subsequent tautomerism and renewed protonation of a formaldehyde molecule can ring in a new condensation reaction and a chain reaction begins.
application
The Baekeland-Manasse-Lederer reaction is particularly important in phenolic resin synthesis.
See also
Individual evidence
- ↑ a b c d A. Baeyer: About the compounds of aldehydes with phenols . In: Reports of the German Chemical Society . tape 5 , no. 1 , 1872, p. 280-282 , doi : 10.1002 / cber.18720050186 .
- ↑ a b L. H. Baekeland: The Synthesis, Constitution, and Uses of Bakelite. In: Journal of Industrial & Engineering Chemistry . tape 1 , no. 3 , 1909, pp. 149-161 , doi : 10.1021 / ie50003a004 .
- ↑ O. Manasse: About a synthesis of aromatic oxyalcohols . In: Reports of the German Chemical Society . tape 27 , no. 2 , 1894, p. 2409-2413 , doi : 10.1002 / cber.189402702239 .
- ↑ L. Lederer: A new synthesis of phenolic alcohols . In: Journal for Practical Chemistry . tape 50 , no. 1 , July 9, 1894, p. 223-226 , doi : 10.1002 / prac.18940500119 .