Barium platinum
| Crystal structure | |||||||
|---|---|---|---|---|---|---|---|
|
|||||||
| __ Ba 2+ __ Pt - | |||||||
| General | |||||||
| Surname | Barium platinum | ||||||
| other names |
Barium monoplatinide |
||||||
| Ratio formula | BaPt | ||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 332.41 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
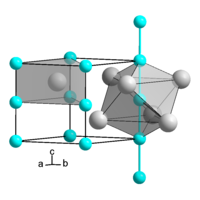
Barium platinum , more precisely barium monoplatinide , BaPt, is a salt of barium with the unusual Pt - ion. In addition to barium monoplatinide, there is a dibariumplatinide and a tribariumdiplatinide .
Extraction and presentation
Barium platinum can be obtained by reacting barium and platinum at around 1000 ° C under an argon atmosphere.
properties
The structure of barium platinum is similar to that of nickel arsenide , but is strongly compressed by the formation of Pt – Pt bonds along the c axis. The bond length between the platinum atoms is 271 pm. The barium gives off a valence electron to the platinum, so the compound is to be formulated as (Ba 2+ ) (Pt - ) · e - . Barium platinum is the first example of a Zintl phase in which the polyatomic structure is established by a transition element .
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Andrey Karpov, Jürgen Nuss u. a .: Covalently Bonded [Pt] - Chains in BaPt: Extension of the Zintl − Klemm Concept to Anionic Transition Metals? In: Journal of the American Chemical Society. Volume 126, 2004, p. 14123, doi: 10.1021 / ja0401186 .
- ^ A b Martin Jansen: Effects of relativistic motion of electrons on the chemistry of gold and platinum . In: Solid State Sciences . tape 7 , December 2005, pp. 1464–1474 , doi : 10.1016 / j.solidstatesciences.2005.06.015 .
- ↑ Maria Barysz, Yasuyuki Ishikawa: Relativistic Methods for Chemists . Springer Science & Business Media, 2010, ISBN 978-1-4020-9975-5 , p. 67 .