Mining-related acidification of ground and surface water
Mining has an impact on water quality due to the lowering of the pH .
Emergence
A problem in ore and coal mining is the formation of acid mine water . These occur when oxygen gets into anoxic mountain areas through mining activities and has an oxidizing effect on the iron disulfide minerals pyrite and marcasite stored there . These oxidations are catalyzed by bacteria and archaea . Abiotic oxidation is very slow. The iron is oxidized to iron (III) ions and released and the sulfide sulfur is oxidized to sulfate ions, with protons H + being released:
- (Eq. 1)
In acidic seepage water, dissolved iron (III) ions can themselves act as oxidizing agents. At pH values above about 2.7 to 3.0, the iron (III) ions combine with water, sulfate ions and possibly with other ions to form iron (III) hydroxide (Fe (OH) 3 ) or jarosites (for example KFe 3 III [(OH) 6 | (SO 4 ) 2 ]) um, whereby protons H + are also released:
- (Eq. 2)
Iron (III) hydroxide and jarosite are sparingly soluble in water and therefore precipitate in the form of a brown precipitate.
Other metal sulphide minerals are also dissolved oxidatively when exposed to oxygen:
- Here Me stands for a divalent metal (e.g. Zn, Pb, Ni, Cu)
In principle, there is no difference between the formation mechanisms in opencast mining and underground operations. A remaining open pit hole then fills with acidic, sulphate and metal-containing water. The possible enrichment of acidic water with (semi-) metal ions is even more problematic in ore mining.
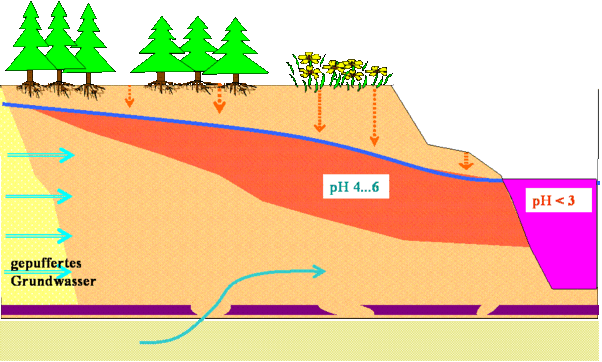
The pH value is not a reliable criterion for the contamination of the water with pyrite weathering products, since mining acidified groundwater and surface water differ from each other, especially in the pH value.
With a lack of oxygen, divalent iron ions (Fe 2+ ) increasingly accumulate , as they are not oxidized to trivalent iron ions due to the lack of oxygen, which is why fewer (protons H +) are released, i.e. less acid is formed.
- (Eq. 3)
Buffering minerals such as carbonates (e.g. calcite , dolomite , siderite ) that are also present in the overburden dissolve by absorbing protons, whereby the seepage water is neutralized, ie the pH value increases.
- (Eq. 4)
Weathering of clay minerals and ion exchange (e.g. H + for Na +) bind further protons formed during pyrite weathering. The quality of the trough's groundwater usually shows a clear vertical structure, which does not always correspond to the geological demarcation of the trough to the grown tertiary aquifer.
Ultimately, the result is an almost oxygen-free groundwater with high sulphate and iron (II) concentrations (in extreme cases> 1000 mg / L in each case) with a pH> 4. The pH value is in the range of unaffected groundwater. The Fe 2+ formed during the weathering of pyrite is a potential acid generator. Unaffected groundwater flows in from outside and from below.
Only after this potentially acidic groundwater has been aerated is the potential acid released through oxidation of the iron (II) and the conversion of the iron (III) formed into iron (III) hydroxide, and the acidic water is created in the open pit lake.
- (Equation 5)
and
- (Eq. 2)
During the oxidation and precipitation of 1 mmol / L or 56 mg / L iron (II) from the groundwater, 2 mmol / L hydrogen ions are formed, the pH value can drop to <2.7. First, the buffering hydrogen carbonate is converted (hydrogen carbonate buffer).
- (Eq. 6)
Iron (III) has limited solubility in water at low pH values and is in chemical equilibrium with precipitated iron hydroxide. Sulphate forms hydrogen sulphate (HSO 4 - ) with the hydrogen ions above pH 1 .
- (Eq. 7)
The maximum pH stabilization is reached around pH ≈ 2.0, where half of the total sulfate is present as hydrogen sulfate.
Consideration of the acidification processes
The neutralization potential
The balance of the acid introduced into the groundwater / seepage water is usually carried out using the acidity concept (Stumm & Morgan 1996). The acidity Aci of an aqueous solution is the excess of strong acids compared to strong bases. Kirby & Cravotta (2005) and Kirby & Cravotta (2005 a) discuss in detail various definitions of acidity and alkalinity. A confusing number of terms results from different titration and calculation methods with different consideration of the dissolved inorganic carbon (DIC) or the cationic acids (Fe, Mn, Al). Therefore, the definition of the neutralization potential (NP) according to Evangelou (1995), modified by Schöpke (1999), is used (all concentrations c in mmol / L):
- (Equation 8)
The acid capacity is titrated with acid or base up to pH = 4.3 ( ) and contains the following ion concentrations:
- (Equation 9)
Since the divalent iron and manganese ions as well as aluminum are not recorded with the acid capacity , they must be taken into account in the calculation. In some cases, other (semi-) metals that hydrolyze up to pH ≈ 7, such as B. Zn, to be considered.
The value of the neutralization potential is the same for tilt groundwater and the seawater formed from it. When iron hydroxide is dissolved / precipitated or iron (2) is oxidized, the pH value is changed, but the acidity of the water, the sum of protons, iron (2) and iron (3), remains the same.
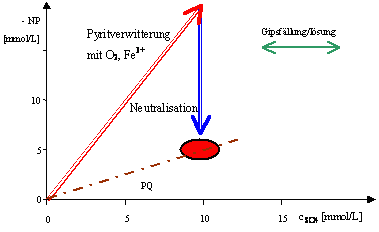
Vector illustration of the formation processes of acidic mining waters
The reactions associated with the genesis of waters influenced by mining can also be shown as vectors in the diagram level of the negative neutralization potential (-NP) versus the sulfate concentration. In the formation of AMD from low-ion precipitation water, the oxidation reactions of the sulphides overlap with buffering reactions and possibly also with the precipitation / dissolution of gypsum. This links the mineral input (main anion sulfate) into the water with the formation of acidity. The negative neutralization potential is shown , which, with the usual indication of acidity , points towards acidification.
The vectors represent changes in concentration. Substance inputs or other reactions can be read quantitatively from their length. Changes in the water quality in this diagram can be traced back to the basic processes shown (pyrite weathering, neutralization and gypsum precipitation / dissolution). The depicted pyrite weathering is a measure of the mining-related acidification:
- Pyrite weathering according to equation 1 and equation 3
- Oxidation with iron (3)
- Eq. (10)
- Oxidation with hydrogen peroxide, for example in the laboratory:
- Eq. (11)
- Oxidation with nitrate:
- Equation (12)
This vector (not drawn) only has a slope of 0.6 (0.6 H + per sulfate). Pyrite oxidation with nitrate can also cause high levels of groundwater iron as a result of overfertilization.
- Entry of solid pyrite weathering products or products adsorbed on the ground
The sulfate concentration is not changed during the neutralization (buffering reactions).
- Calcite conversion with acid according to equation 4
- Adding lye
- Eq. (13) or
- Equation (14)
Reactions that do not change the neutralization potential (acidity):
- Oxidation of iron (2) according to equation 5
- Reductive solution of iron hydroxide, e.g. B. through carbohydrates
- Eq. (15)
- Precipitation / dissolution of iron (3) hydroxide according to equation 2
- Precipitation / dissolution of iron carbonate (siderite)
- Eq. (16)
- Precipitation / dissolution of aluminum hydroxide
- Equation (17)
When iron (II) is oxidized by oxygen, the neutralization potential does not change by definition. This also applies to the reverse reaction with oxygen-consuming substances according to Eq. 15. Mineral phases in equilibrium with the iron species according to Eq. 5, Eq. 16 and Eq. 17 also do not change the acidity, but do change the pH value. These reactions do not appear in the vector diagram or, strictly speaking, represent a point. However, the exact composition of mining acidified and partially neutralized water is characterized by a large number of reactions that do not affect the neutralization potential or the sulphate concentration. The vector illustration presented allows all processes that are less important for the assessment of mining acidification to be hidden. Tilt groundwater and the associated acidic lake water with different properties are congruent. In this way, for example, renovation measures can be prepared in a targeted manner.
The hydrochemical details have to be developed step by step using geochemical modeling, for example with PHREEQC according to Parkhurst & Appelo (2006).
Analysis examples
The compositions of groundwater and surface water vary within wide limits. To illustrate this, data from Lausitzer Wässern (Schöpke 1999) and mine water from underground mining (Gammons et al. 2006) are compiled:
| parameter | unit | Groundwater | Tilt groundwater | Open pit lake | Pit water |
|---|---|---|---|---|---|
| pH | 1 | 7th | 4.5 ... 6.4 | 2.8 | 4.6 |
| electric conductivity | µS / cm | 400 | 800 ... 3000 | 2900 | 6200 |
| K S4.3 | mmol / l | 1.6 | 0.5 ... 4 | −3.8 | |
| Approx | mg / L | 80 | 140 ... 700 | 300 | 490 |
| Mg | mg / L | 6th | 11 ... 50 | 24 | 400 |
| Fe | mg / L | 12 | 50 ... 2000 | 130 | 1770 |
| SO 4 2− | mg / L | 100 | 500 ... 3600 | 1300 | 7700 |
| Neutralization potential (NP) | mmol / L | > 0.5 | −70… 0 | −11 | −83 |
The pit water from underground mining has very high concentrations of manganese, aluminum and (semi-) metals, which have been taken into account when calculating the neutralization potential.
- Genesis of Kippen groundwater
- Presentation of the hydrochemistry of remedial measures
See also
literature
- VP Evangelou: Pyrite oxidation and its control. CRC Press, Boca Raton / New York / London / Tokyo 1995, ISBN 0-8493-4732-7 .
- CH Gammons, JJ Metesh, DM Snyder: A Survey of the Geochemistry of Flooded Mine Shaft Water in Butte, Montana. In: Mine Water and the Environment. 25, No. 2, 2006, pp. 100-107.
- CS Kirby, CA Cravotta: Net alkalinity and net acidity 2: PRACTICAL considerations. In: Applied Geochemistry. Vol. 20, No. 10, 2005, pp. 1941-1964.
- CS Kirby, CA Cravotta: Net alkalinity and net acidity 1: Theoretical considerations. In: Applied Geochemistry. Vol. 20, No. 10 2005, pp. 1920-1940.
- DL Parkhurst, CAJ Appelo: PHREEQC Version 3. (= Water-Resources Investigations Report 99-4259). 2006.
- R. Schöpke: Development of a method to describe hydrochemical processes in tipping aquifers. (= Urban water management and the environment. Issue 2).
- R. Schöpke, W. Pietsch: Chemically induced changes in the properties of seepage and groundwater (final report, subproject 10). In: BTUC: Innovationskolleg. Ecological development potential of the post-mining landscape in the Lusatian lignite district. BG Teubner Stuttgart / Leipzig / Wiesbaden 2000, ISBN 3-519-00321-X .
- W. Stumm, JJ Morgan: Aquatic chemistry - Chemical Equilibria and Rates in Natural Waters. 3. Edition. John Wiley, New York 1996, ISBN 0-471-51184-6 .
- Ch.Wolkersdorfer: Water Management at Abandoned Flooded Underground Mines - Fundamentals, Tracer Tests, Modeling, Water Treatment. Springer, Berlin 2008, ISBN 978-3-540-77330-6 .
- PL Younger, SA Banwart, RS Hedin: Mine Water - Hydrology, Pollution, Remediation. Kluwer, Dordrecht 2002, ISBN 1-4020-0137-1 .
- PL Younger, NS Robins: Mine Water Hydrogeology and Geochemistry. (= Spec. Publ. - Geol. Soc. London. 198). London 2002, ISBN 1-86239-113-0 .
Web links
- Lusatian and Central German Mining Management Company Ltd.
- Dresden Groundwater Research Center (DGFZ)
- International Mine Water Association (IMWA)
- Mine Water and the Environment magazine
- Publications of the Department of Water Technology & Sanitary Engineering of the BTU Cottbus
Individual evidence
- ↑ (usgs.gov)
- ↑ Brandenburg University of Technology Cottbus ( Memento from March 4, 2016 in the Internet Archive ) (PDF file; 2.82 MB). Dissertation BTU Cottbus LS water technology, 1999.


















![{\ displaystyle Fe (OH) _ {3} +2 \, H ^ {+} + 0.25 \, [CH_ {2} O] \ rightarrow Fe ^ {2 +} + 0.25 \, CO_ {2 } +2.5 \, H_ {2} O}](https://wikimedia.org/api/rest_v1/media/math/render/svg/302d3316d0d68a624c62457857ce1204abe3d8c7)

