Blue and white selection
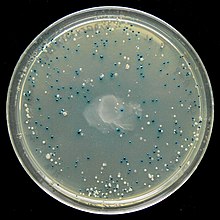
The blue white screen is a method of genetic engineering for identification of bacterial clones containing a transgene contained.
principle
The aim of the blue-white selection is to identify transgenic bacteria with the desired modifications. On the one hand, those bacteria are identified that have taken up a plasmid (usually a cloning vector ) and, on the other hand, the blue-white selection answers the question of whether the plasmid was successfully modified. For the determination of plasmid-containing bacteria, the plasmids carry a gene which confers an antibiotic resistance that the bacterial strain used does not already possess. If the bacteria are cultivated on a culture medium with the antibiotic suitable for the resistance, ideally only those bacteria survive there that have ingested a plasmid.
For the blue-white selection, certain plasmids are used as vectors which contain the gene for a β-galactosidase ( lacZ gene) at the position for the insertion of the transgene into the plasmid (at the multiple cloning site ) . The gene for galactosidase is used as a reporter gene .
The galactosidase is inactivated by inserting a transgene into the multiple cloning site. As a result, after a transformation of the plasmids, the transgenic organisms, in contrast to the non-transgenic organisms, do not contain any functional galactosidase, provided that the bacterial strain used for the transformation does not contain a further gene for a galactosidase. For this purpose, special mutant strains are used (e.g. E. coli strains JM109 , DH5α and XL1-Blue ) which contain a mutation (e.g. lacZΔM15 , from E. coli M15 ) which suppresses the expression of a functional galactosidase. The lacZΔM15 mutant of galactosidase has a deletion of amino acids 11 to 41, which means that galactosidase can no longer form a homotetramer , which is the enzymatically active form of galactosidase. The lacZΔM15 mutant of galactosidase (synonymous ω-peptide) can, however, be supplemented to a functional form by a protein of amino acids 1 to 59 of galactosidase (α-peptide), the gene of which is located on the plasmid in this case.
The galactosidase is a glycosidase and the yellow dye can XGal in a blue dye (5,5'-dibromo-4,4'-dichloro-indigo) and galactose split . The gene lacZ of galactosidase is from a promoter controlled, wherein the gene expression by an inducer (eg. Lactose , allolactose , the strongest known inducer is IPTG is initiated because the) lacZ - repressor LacI by binding of the inducer the gene for Releases gene expression. When using XGal in the culture medium, a blue dye is produced about one hour after induction by the galactosidase contained in the bacteria. In the case of the transgenic organisms, on the other hand, the galactosidase is inactivated, which means that their colonies remain unstained and they can be isolated on the basis of their lack of color. Since the import of lactose via the lactose permease (gene lacY ) is inhibited by glucose , the culture medium should not contain any glucose.
Not every white colony is a transgenic organism (e.g. because of LacZ inactivation by DNA repair of non- ligated plasmids) and not every transgenic organism contains the desired gene to be cloned, but other DNA fragments. If too little antibiotic or too old culture media are used to select for the resistance gene of the plasmid, white colonies without plasmid also grow. For these reasons, a further analysis is carried out, e.g. B. by polymerase chain reaction (mostly colony polymerase chain reaction ) or by DNA extraction with restriction digestion , both with subsequent agarose gel electrophoresis . XGal is photosensitive, which is why the dye itself and agar plates containing XGal are stored in the dark.
history
Blue-white selection was first used in complementation experiments; H. the function of the galactosidase should be restored by inserting a previously removed piece of DNA. The blue-white selection for cloning purposes was used for the first time in pUC plasmids (e.g. pUC19 ).
literature
- Cornel Mülhardt: The Experimenter: Molecular Biology / Genomics. Sixth edition. Spectrum Akademischer Verlag, Heidelberg 2008. ISBN 3-8274-2036-9 .
Individual evidence
- ↑ M. Koenen, U. Rüther, B. Müller-Hill: Immunoenzymatic detection of expressed gene fragments cloned in the lac Z gene of E. coli. In: The EMBO journal. Volume 1, Number 4, 1982, ISSN 0261-4189 , pp. 509-512, PMID 6329687 , PMC 553076 (free full text).
- ^ A. Ullmann, Francois Jacob , Jacques Monod : Characterization by in vitro complementation of a peptide corresponding to an operator-proximal segment of the beta-galactosidase structural gene of Escherichia coli. In: Journal of molecular biology. Volume 24, Number 2, March 1967, ISSN 0022-2836 , pp. 339-343, PMID 5339877 .
- ↑ KE Langley, MR Villarejo, AV Fowler, PJ Zamenhof, I. Zabin: Molecular basis of beta-galactosidase alpha-complementation. In: Proceedings of the National Academy of Sciences . Volume 72, Number 4, April 1975, ISSN 0027-8424 , pp. 1254-1257, PMID 1093175 , PMC 432510 (free full text).
- ↑ J. Messing, B. Gronenborn, B. Müller-Hill, P. Hans Hopschneider: Filamentous coliphage M13 as a cloning vehicle: insertion of a HindII fragment of the lac regulatory region in M13 replicative form in vitro. In: Proceedings of the National Academy of Sciences . Volume 74, Number 9, September 1977, ISSN 0027-8424 , pp. 3642-3646, PMID 333444 , PMC 431673 (free full text).
- ↑ J. Vieira, J. Messing: The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. In: Genes. Volume 19, Number 3, October 1982, ISSN 0378-1119 , pp. 259-268, PMID 6295879 .
