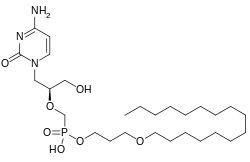
Brincidofovir
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Brincidofovir | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 27 H 52 N 3 O 7 P | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 561.70 g mol −1 | |||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Brincidofovir (synonym CMX001 ) is an experimental antiviral agent against various viruses and a derivative of the antiviral agent cidofovir . Brincidofovir was used in humans during the Ebola virus epidemic in 2014 without the usually required drug approval .
properties
In animal experiments, brincidofovir is effective against the herpes simplex virus 1 , adenovirus (AdV), BK virus , smallpox viruses and the Ebola virus . In comparison to cidofovir, brincidofovir was extended by a lipid that is split off within the cell and is metabolized to the active ingredient cidofovir diphosphate. Brincidofovir was originally developed for use in infections with DNA viruses , but it is also effective against Ebola virus. Brincidofovir was developed by the Chimerix company.
In the wake of the Ebola virus epidemic in 2014, the Food and Drug Administration initiated an accelerated approval process for brincidofovir and it was investigated in clinical phase II studies from October 2014 . The World Health Organization wrote in a statement that in the course of the Ebola virus epidemic in 2014, it was ethically acceptable to use preventive or therapeutic drugs without proof of effectiveness in humans, if promising results could be shown in animal experiments.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b D. F. Florescu, MA Keck: Development of CMX001 (Brincidofovir) for the treatment of serious diseases or conditions caused by dsDNA viruses. In: Expert Review of Anti-Infective Therapy . Volume 12, number 10, October 2014, pp. 1171–1178, doi : 10.1586 / 14787210.2014.948847 . PMID 25120093 .
- ↑ a b Chimerix Announces Emergency Investigational New Drug Applications for Brincidofovir Authorized by FDA for Patients With Ebola Virus Disease . Archived from the original on October 8, 2014. Retrieved October 8, 2014.
- ↑ DC Quenelle, B. Lampert, DJ Collins, TL Rice, GR Painter, ER Kern: Efficacy of CMX001 against herpes simplex virus infections in mice and correlations with drug distribution studies. In: The Journal of Infectious Diseases . Volume 202, Number 10, November 2010, pp. 1492-1499, doi : 10.1086 / 656717 . PMID 20923374 . PMC 2957530 (free full text).
- ↑ AE Tollefson, JF Spencer, B. Ying, RM Buller, WS Wold, K. Toth: Cidofovir and brincidofovir reduce the pathology caused by systemic infection with human type 5 adenovirus in immunosuppressed Syrian hamsters, while ribavirin is largely ineffective in this model. In: Antiviral Research . Volume 112, December 2014, pp. 38-46, doi : 10.1016 / j.antiviral.2014.10.005 . PMID 25453340 .
- ↑ DF Smee, A. Dagley, B. Downs, J. Hagloch, EB Tarbet: Enhanced efficacy of cidofovir combined with vaccinia immune globulin in treating progressive cutaneous vaccinia virus infections in immunosuppressed hairless mice. In: Antimicrobial Agents and Chemotherapy . [electronic publication before printing] November 2014, doi : 10.1128 / AAC.04289-14 . PMID 25385098 .
- ↑ a b David Kroll: Chimerix's Brincidofovir Given To Dallas, Nebraska Ebola Patients . forbes.com. October 7, 2014. Retrieved January 14, 2015.
- ↑ DF Florescu, MA Keck: Development of CMX001 (Brincidofovir) for the treatment of serious diseases or conditions caused by dsDNA viruses. In: Expert review of anti-infective therapy. Volume 12, number 10, October 2014, pp. 1171–1178, doi : 10.1586 / 14787210.2014.948847 . PMID 25120093 .
- ↑ Wall Street Journal: Chimerix to conduct Ebola Drug Trial: Drug Company Gets FDA Approval to Start Trial Immediately in Infected Patients .
- ↑ WHO - Ethical considerations for use of unregistered interventions for Ebola virus disease . Retrieved October 8, 2014.