Butanals
Butanals are aldehydes with four carbon atoms. They have the general molecular formula C 4 H 8 O and a molar mass of 72.11 g / mol. There are only two isomers :
| Butanals | ||
| Surname | n -butanal | Isobutanal |
| other names | n -Butyraldehyde | Isobutyraldehyde |
| Structural formula | 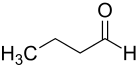 |
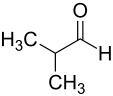
|
| CAS number | 123-72-8 | 78-84-2 |
| PubChem | 261 | 6561 |
| Molecular formula | C 4 H 8 O | |
| Molar mass | 72.11 g mol −1 | |
| Physical state | liquid | |
| Melting point | −91.7 ° C | −65 ° C |
| boiling point | 75 ° C | 64 ° C |
Crotyl alcohol , 3-buten-1-ol and butanone also have the same empirical formula .
Individual evidence
- ↑ a b Entry on butyraldehyde in the GESTIS substance database of the IFA , accessed on May 24, 2016(JavaScript required) .
- ↑ a b Entry on isobutyraldehyde in the GESTIS substance database of the IFA , accessed on May 24, 2016(JavaScript required) .