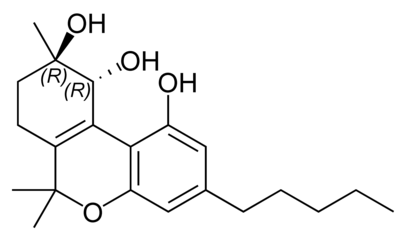
Cannabitriol
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
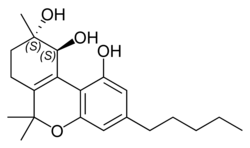
|
||||||||||
| General | ||||||||||
| Surname | Cannabitriol | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 21 H 30 O 4 | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| Drug information | ||||||||||
| Drug class | ||||||||||
| properties | ||||||||||
| Molar mass | 346.46 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
171-173 ° C |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Cannabitriol ( CBT ) is a phytocannabinoid from the hemp plant ( cannabis ) that was first isolated and described in 1966 by Yataro Obata and Yoshinori Ishikawa.
chemistry
Cannabitriol has essentially the same basic structure as tetrahydrocannabinol (Δ 9 -THC), but has two additional alcoholic hydroxyl groups and the position of the double bond differs from that in Δ 9 -THC.
Individual evidence
- ↑ Burkhard Fugmann, Susanne Lang-Fugmann, Wolfgang Steglich: RÖMPP Encyclopedia Natural Products, 1st Edition, 2000 . Georg Thieme Verlag, 2014, ISBN 3-13-179311-2 , p. 108 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ A b W. R. Chan, KE Magnus, HA Watson: The structure of cannabitriol. In: Experientia. 32, 1976, p. 283, doi : 10.1007 / bf01940792 .
- ↑ Yataro Obata & Yoshinori Ishikawa (1966): Studies on the Constituents of Hemp Plant (Cannabis sativa L.) , Agricultural and Biological Chemistry, 30: 6, 619–620, doi : 10.1080 / 00021369.1966.10858651 .
Web links
- Marijuana and the Cannabinoids , p. 24