Chloroacrylic acids
| Monochloroacrylic acids | ||||
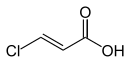
| Surname | 2-chloroacrylic acid | cis -3-chloroacrylic acid | trans -3-chloroacrylic acid | |
| Structural formula |  |
 |
|
|
| CAS number | 598-79-8 | 1609-93-4 | 2345-61-1 | |
| PubChem | 11735 | 643794 | 638124 | |
| Molecular formula | C 3 H 3 ClO 2 | |||
| Molar mass | 106.51 g mol −1 | |||
| Physical state | firmly | |||
| description | ||||
| Melting point | 63-67 ° C | 60-61 ° C | 81-85 ° C | |
|
GHS labeling |
|
|||
| H and P phrases | 314-318 | |||
| no EUH phrases | ||||
|
260-303 + 361 + 353-305 + 351 + 338 301 + 330 + 331-405-501 |
||||
The chloroacrylic acids form a group of substances in chemistry and are derived from acrylic acid .
Manufacturing
2-chloroacrylic acid can be prepared from 2,3-dichloropropionic acid with potassium ethoxide .
3-chloroacrylic acid is formed from propargylic acid and hydrogen chloride .
Individual evidence
- ↑ a b Data sheet 2-Chloroacrylic acid from AlfaAesar, accessed on January 28, 2014 ( PDF )(JavaScript required) .
- ↑ a b Data sheet cis-3-Chloroacrylic acid from AlfaAesar, accessed on January 28, 2014 ( PDF )(JavaScript required) .
- ↑ a b Data sheet trans-3-Chloroacrylic acid from AlfaAesar, accessed on January 28, 2014 ( PDF )(JavaScript required) .
- ↑ Werigo, Melikoff: "Ueber Bichlorpropionsäure aus Glycerinsäure", in: Reports of the German Chemical Society , 1877 , 10 (2), pp. 1499-1500 ( doi : 10.1002 / cber.18770100253 ).
- ↑ E. Baudrowski: "Ueber Propargyläure (C 3 H 2 O 2 )", in: Reports of the German Chemical Society , 1882 , 15 (2), pp. 2698-2704 ( doi : 10.1002 / cber.188201502243 ).
