Chloryl fluoride
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
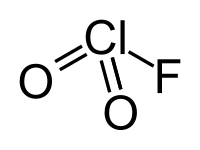
|
||||||||||
| General | ||||||||||
| Surname | Chloryl fluoride | |||||||||
| other names |
Chlorine dioxide fluoride |
|||||||||
| Molecular formula | ClFO 2 | |||||||||
| Brief description |
colorless gas |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 86.45 g mol −1 | |||||||||
| Physical state |
gaseous |
|||||||||
| boiling point |
−6 ° C |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Chloryl fluoride is a chemical compound from the group of fluorides in chloric acid .
Extraction and presentation
Chloryl fluoride can be obtained by reacting chlorine dioxide with fluorine at around −50 ° C.
It was first synthesized by Schmitz and Schumacher in 1942.
It can also be represented by the reaction of potassium chlorate or sodium chlorate with chlorine trifluoride .
properties
Chloryl fluoride is a colorless gas that is very sensitive to moisture. It immediately forms fog in moist air. The compound is much more thermally stable than chlorine dioxide.
Individual evidence
- ↑ a b c d e Georg Brauer (Ed.), With the collaboration of Marianne Baudler u. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , p. 166.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ H. Harry Julius Emeleus, AG Sharpe: Advances in Inorganic Chemistry and Radiochemistry . Academic Press, 1976, ISBN 0-08-057867-5 , pp. 347 ( limited preview in Google Book search).
- ^ AF Holleman, Egon Wiberg, Nils Wiberg: Inorganic Chemistry . Academic Press, 2001, ISBN 0-12-352651-5 , pp. 1797 ( limited preview in Google Book search).

