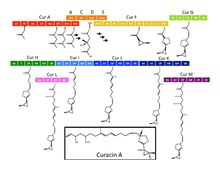
Curacin A
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||
| General | |||||||||||||
| Surname | Curacin A | ||||||||||||
| Molecular formula | C 23 H 35 NOS | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 373.60 g mol −1 | ||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Curacin A is a natural product isolated from the cyanobacterium Lyngbya majuscula . Curacin A, along with jamaicamide , mupirocin and pederin, belongs to a family of natural substances that have a terminal alkene . In addition, curacin A contains a thiazoline ring and a cyclopropyl moiety , which is essential for the biological activity of the compound. Curacin A has been characterized as a potent, growth-inhibiting, cytotoxic compound with remarkable anticancer activity for a variety of cancer lines including kidney , colon and breast cancers . Curacin A has been shown to interact with colchicine binding sites on tubulin , which inhibits microtubule polymerization, an essential process for cell division and growth.
Isomers
Curacin B and C are isomers of curacin A. Curacin D is probably demethylcuracin.
biosynthesis
The biosynthesis of curacin A has been carried out by Gu et al. fully described. The synthesis is based on a gene cluster of approximately 64 kb that express NRPS and several polyketide synthases (PKSs).
Legal position
Curacin A and its isomers have been patented since 1998.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b c d Zunxue Chang, Namthip Sitachitta, James V. Rossi, Mary Ann Roberts, Patricia M. Flatt, Junyong Jia, David H. Sherman, William H. Gerwick: Biosynthetic Pathway and Gene Cluster Analysis of Curacin A, an Antitubulin Natural Product from the Tropical Marine Cyanobacterium . In: Journal of Natural Products . 67, No. 8, August 2004, pp. 1356-1367. doi : 10.1021 / np0499261 . PMID 15332855 .
- ↑ a b c d e Jean-Michel Kornprobst: Encyclopedia of Marine Natural Products . Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany 2014, ISBN 978-3-527-33585-5 , doi : 10.1002 / 9783527335855 ( wiley.com [accessed June 11, 2019]).
- ↑ a b Liangcai Gu, Todd W. Geders, Bo Wang, William H. Gerwick, Kristina Håkansson, Janet L. Smith, David H. Sherman: GNAT-Like Strategy for Polyketide Chain Initiation . In: Science . 318, 2007, pp. 970-974. doi : 10.1126 / science.1148790 . PMID 17991863 .
- ↑ AV Blokhin, HD Yoo, RS Geralds, DG Nagle, WH Gerwick, E. Hamel: Characterization of the interaction of the marine cyanobacterial natural product curacin A with the colchicine site of tubulin and initial structure-activity studies with analogues . In: Molecular Pharmacology . 48, 1995, pp. 523-531. PMID 7565634 .