Curium (III) oxalate
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
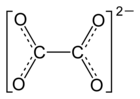
|
|||||||
| General | |||||||
| Surname | Curium (III) oxalate | ||||||
| Molecular formula |
|
||||||
| Brief description |
light green solid |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | (for 248 cm): 940.22 g mol −1 (decahydrate) | ||||||
| Physical state |
firmly |
||||||
| solubility |
soluble in aqueous alkali metal carbonate solutions |
||||||
| Hazard and safety information | |||||||
 Radioactive |
|||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Curium (III) oxalate is the oxalic acid salt of the element curium . The decahydrate has the empirical formula Cm 2 (C 2 O 4 ) 3 · 10 H 2 O.
presentation
Curium (III) oxalate is formed when aqueous curium (III) solutions are mixed with oxalic acid.
properties
The decahydrate gradually dehydrates in a vacuum , changing to anhydrous form at 280 ° C. If it is further heated to 360 ° C, the carbonate is formed .
The hydrated oxalate is soluble in aqueous alkali metal carbonate solutions. The solubility increases sharply in the heat.
Due to the high level of radioactivity, freshly filtered 244 curium oxalate is subject to radiolysis within a few hours with formation of the carbonate, which could be demonstrated by the evolution of gas after dissolving in acid.
use
Curium oxalate is routinely used to prepare curium (IV) oxide (CmO 2 ) over curium hydroxide (Cm (OH) 3 ).
Furthermore, curium ions, which have been contaminated with plutonium and americium due to long storage , can be recovered from their solutions in very high yields (up to 99.4%) by oxalate precipitation.
safety instructions
Classifications according to the CLP regulation are not available, as this only relates to chemical hazard. Curium and its compounds are highly radioactive and must therefore be handled with the greatest care.
literature
- Gregg J. Lumetta, Major C. Thompson, Robert A. Penneman, P. Gary Eller: Curium , in: Lester R. Morss, Norman M. Edelstein, Jean Fuger (Eds.): The Chemistry of the Actinide and Transactinide Elements , Springer, Dordrecht 2006; ISBN 1-4020-3555-1 , pp. 1397-1443 ( doi : 10.1007 / 1-4020-3598-5_9 ).
Individual evidence
- ↑ a b G. A. Burney, JA Porter: Solubilities of Pu (III), Am (III), and Cm (III) oxalates , in: Inorganic and Nuclear Chemistry Letters , 1967 , 3 (3), pp. 79-85 ( doi : 10.1016 / 0020-1650 (67) 80128-4 ).
- ↑ a b N. E. Bibler: Hydroxide metathesis of multigram amounts of curium oxalate , in: Inorganic and Nuclear Chemistry Letters , 1972 , 8 (2), pp. 153–156 ( doi : 10.1016 / 0020-1650 (72) 80102-8 ) .
- ↑ The hazards emanating from radioactivity do not belong to the properties to be classified according to the GHS labeling. With regard to other hazards, this substance has either not yet been classified or a reliable and citable source has not yet been found.
- ↑ Gregg J. Lumetta, Major C. Thompson, Robert A. Penneman, P. Gary Eller: Curium , in: Lester R. Morss, Norman M. Edelstein, Jean Fuger (ed.): The Chemistry of the actinides and transactinide element element , Springer, Dordrecht 2006; ISBN 1-4020-3555-1 , pp. 1397-1443 ( doi : 10.1007 / 1-4020-3598-5_9 ).
- ↑ a b V. Scherer, M. Fochler: The thermal decomposition of curium (III) oxalate IO-hydrate , in: Journal of Inorganic and Nuclear Chemistry , 1968 , 30 (6), pp. 1433-1437 ( doi : 10.1016 / 0022-1902 (68) 80282-9 ).
- ↑ Hirokazu Hayashi, Hiromichi Hagiya, Seong-Yun Kim, Yasuji Morita, Mitsuo Akabori, Kazuo Minato: Separation and recovery of Cm from Cm – Pu mixed oxide samples containing Am impurity , in: Journal of Radioanalytical and Nuclear Chemistry , 2013 , 296 ( 3), pp. 1275-1286 ( doi : 10.1007 / s10967-012-2304-y ).


