Cytochrome P450 2R1
| Cytochrome P450 2R1 | ||
|---|---|---|
|
||
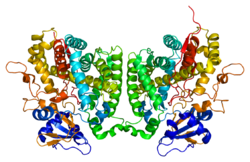
| Crystal structure of cytochrome P450 2R1 according to PDB 2ojd | ||
| Properties of human protein | ||
| Mass / length primary structure | 501 amino acids | |
| Cofactor | Heme thiolate | |
| Identifier | ||
| Gene name | CYP2R1 | |
| External IDs | ||
| Enzyme classification | ||
| EC, category | 1.14.13.15 , oxygenase | |
| Response type | Hydroxylation | |
| Substrate | 3α, 7α, 12α-trihydroxy-5β-cholestane | |
| Products | 3α, 7α, 12α, 26-tetrahydroxy-5β-cholestane | |
| Occurrence | ||
| Parent taxon | Vertebrates | |
Cytochrome P450 2R1 (CYP2R1) (also: vitamin D - 25-hydroxylase ) is the enzyme that hydroxylates vitamin D to calcidiol . This response is the first of two in the biosynthesis of the hormone calcitriol ; in all vertebrates, including humans, it takes place exclusively in the microsomes of the liver . Mutations in CYP2R1 - gene can enzyme deficiency, and this to a rare inherited form of rickets lead.
In a study of 200 diabetes 1 patients, CYP2R1 variants were associated with low vitamin D levels.
Catalyzed reaction
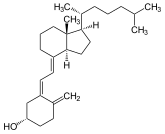 + NADPH / H + + O 2 ⇒
+ NADPH / H + + O 2 ⇒
⇒  + NADP + + H 2 O
+ NADP + + H 2 O
Vitamin D3 is hydroxylated to calcidiol. Vitamin D2 is also accepted as a substrate .
Individual evidence
- ↑ Nelson DR: Comparison of P450s from human and fugu: 420 million years of vertebrate P450 evolution . In: Arch. Biochem. Biophys. . 409, No. 1, January 2003, pp. 18-24. PMID 12464240 .
- ↑ UniProt Q6VVX0
- ↑ Ramos-Lopez E, Brück P, Jansen T, Herwig J, Badenhoop K: CYP2R1 (vitamin D 25-hydroxylase) gene is associated with susceptibility to type 1 diabetes and vitamin D levels in Germans . In: Diabetes Metab. Res. Rev. . 23, No. 8, November 2007, pp. 631-6. doi : 10.1002 / dmrr.719 . PMID 17607662 .
Web links
Wikibooks: Biochemistry and Pathobiochemistry: Vitamin D Metabolism - Learning and Teaching Materials
- Jassal / D'Eustachio / reactome.org: 25-Hydroxylation of vitamin D3 in liver