Daxalipram
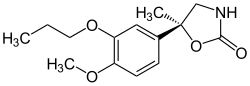
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| ( R ) -daxalipram (top) and ( S ) -daxalipram (bottom) | |||||||||||||
| General | |||||||||||||
| Non-proprietary name | Daxalipram | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 14 H 19 NO 4 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| Drug class | |||||||||||||
| Mechanism of action | |||||||||||||
| properties | |||||||||||||
| Molar mass | 265.31 g mol −1 | ||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Daxalipram is an experimental drug that is believed to have effects in multiple sclerosis and colitis . It is an inhibitor of phosphodiesterase-4 (PDE-4) and reduces the breakdown of the secondary messenger substance cAMP by intracellular phosphodiesterases .
Daxalipram is a γ- lactam and was first mentioned in a Schering AG patent in 1997 and described in the literature as mesopram in 2000 .
Pharmacological properties
Mechanism of action
Daxalipram inhibits the isoenzymes B and D of PDE-4 . It has little or no effect on other phosphodiesterases.
Anti-inflammatory effects
Based on animal experiments, Daxalipram is ascribed anti-inflammatory effects, especially in multiple sclerosis . Daxalipram is said to reduce the degree of disease of experimental brain inflammation in rodents and to suppress the migration of inflammatory cells into the nervous system and the production of cytokines in a dose-dependent manner.
More recent studies show that the anti-inflammatory effects of daxalipram in the brain cannot be traced back to a stabilization of the blood-brain barrier .
Daxalipram was also effective against inflammatory bowel disease in a mouse model .
Other effects
Daxalipram was able to induce ovulation in rats . This could make daxalipram (or other phosphodiesterase-4 inhibitors) an alternative to injection treatment for infertility .
Clinical effects
Daxalipram was developed by Schering AG u. a. designed to treat multiple sclerosis . However, it has not yet been approved as a medicinal product. The development was stopped in 2004 due to » prioritization of other development projects«.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Laurent H, Ottow E, Kirsch G et al. Chiral methyl phenyl oxazolidinones. 1997; WO 97/15561.
- ↑ a b Dinter H, Tse J, Halks-Miller M et al. The type IV phosphodiesterase specific inhibitor mesopram inhibits experimental autoimmune encephalomyelitis in rodents. J Neuroimmunol 2000; 108: 136-146, PMID 10900347 .
- ↑ a b Card GL, England BP, Suzuki Y et al. Structural basis for the activity of drugs that inhibit phosphodiesterases. Structure. 2004; 12: 2233-47, PMID 15576036 .
- ^ Schmid H. The effect of the phosphodiesterase IV inhibitor mesopram on factors of the blood-brain barrier. Inaug Diss; Würzburg 2008 (PDF; 725 kB).
- ↑ Loher F, Schmall K, Freytag P et al .: The specific type-4 phosphodiesterase inhibitor mesopram alleviates experimental colitis in mice. J Pharmacol Exp Ther . 2003; 305: 549-56. PMID 12606674
- ↑ McKenna SD, Pietropaolo M, Tos EG et al .: Pharmacological inhibition of phosphodiesterase 4 triggers ovulation in follicle-stimulating hormone-primed rats. Endocrinology. 2005; 146: 208-14, PMID 15448112 .
- ↑ Schering AG: 2004 Annual Report on Form 20-F , accessed on August 17, 2008.