Dimedon
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 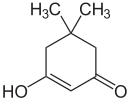
|
||||||||||||||||
| Keto form (left) and enol form (right) | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Dimedon | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 8 H 12 O 2 | |||||||||||||||
| Brief description |
white crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 140.18 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
146-148 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Dimedon is used in organic analysis to identify and separate aldehydes (including formaldehyde ). It forms white crystals.
presentation
The presentation is achieved by deprotonation of diethyl malonate in a sodium ethoxide . Mesityloxide is added in a slight deficit . After prolonged heating, it is neutralized, with the dimedon separating out as an oil or even in crystalline form.
properties
Dimedon stands in solution in a tautomeric equilibrium - in the ratio 2: 1 of the keto / enol form in chloroform .
Individual evidence
- ↑ a b R. L. Shriner, HR Todd: 5,5-Dimethylcyclohexane-1,3-dione In: Organic Syntheses . 15, 1935, p. 14, doi : 10.15227 / orgsyn.015.0014 ; Coll. Vol. 2, 1943, p. 200 ( PDF ).
- ↑ a b c data sheet 5,5-dimethyl-1,3-cyclohexanedione from Sigma-Aldrich , accessed on May 12, 2017 ( PDF ).
- ↑ Jonathan Clayden, Nick Greeves, Stuart Warren, Peter Wothers: Organic Chemistry , 2001, Oxford University Press, ISBN 0-19-850346-6 , p. 532: Formation and reactions of enols and enolates.
- ↑ M. Bolte, M. Scholtyssik: Dimedone at 133K , in: Acta Cryst. , 1997 , C53 , IUC9700013; doi : 10.1107 / S0108270197099423 .
