Dipole-Dipole Forces
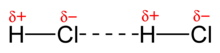
As dipole-dipole forces (including dipole-dipole interactions , Keesom forces or Keesom interactions called) are referred to the forces that exist between molecules, which a permanent electric dipole moment own (for the magnetic dipole-dipole interaction, e.g. B. in a paramagnetic or ferromagnetic solid, the same applies). The strength depends on the distance and relative orientation of the dipole.
These intermolecular forces are weaker than the forces in the hydrogen bonds , but stronger than the London forces ( Van der Waals forces in the narrower sense ).
In the case of atomic bonds within molecules, a distinction is made between covalent or non-polar bonds (difference in electronegativity is 0.0–0.4) and polar bonds (difference in electronegativity is 0.4–1.7) on the basis of the difference in electronegativity .
In the case of polar bonds, the bond electrons are attracted to the more electronegative atom. This gives the molecule a negative and a positive partial charge. The water molecule has an intrinsic dipole moment, but the CH 4 molecule does not. However, there is only a dipole when an equivalent vector can be formed. It is also said that the “negative and positive centers of charge do not coincide on one point”.
Individual evidence
- ↑ Entry on dipole-dipole-interactions . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.D01758 Version: 2.1.5.