Single molecule fluorescence spectroscopy
The single molecule fluorescence spectroscopy ( English Single Molecule Fluorescence Spectroscopy ) is a method of physical chemistry , to make individual molecules visible. As a rule, a confocal microscope or a TIRF microscope is used, which allows the size of the detection volume to be limited to less than one femtoliter ( 10-15 liters). This means that, given suitable dilution of the molecules to be examined, there is on average less than one fluorescence-active molecule in focus. After excitation by a suitable laser , the excited molecule emits photons ( fluorescence ), which can be used to characterize the properties of individual molecules. In the case of ensemble measurements, on the other hand, there are several molecules in the observation volume, so that the individual molecule properties remain hidden and only an average value is detected.
Ensemble vs. Single molecule measurements
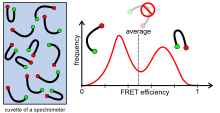
Ensemble measurements (e.g. in a fluorescence or absorption spectrometer) always result in a mean value averaged over the entire sample. However, this can simulate a sample that is not actually there, because z. B. the mean over two different species in the sample gives the same value.
An example: If you measure the total fluorescence intensity of a sample with, for example, ten fluorescence-active molecules during an ensemble measurement, you get a certain time average. However, this hides the fact that six of the ten molecules may only fluoresce weakly and the other four make up the main part of the measured fluorescence intensity. The same is shown in the figure on the right for a FRET measurement. The sample consists of two different conformations that give either a high or a low FRET. Averaging over both would result in a mean FRET, according to which one would infer a third conformation that is actually not present.
Procedure for the measurement
Diffusion measurements or flow-driven measurements
The molecules to be examined are diluted in a buffer solution and introduced into the microscope in such a way that the probability of encountering two fluorescent molecules in the observation volume at the same time is negligible. During a longer measurement (typically 10–60 min) that follows, individual molecules diffuse one after the other through the observation volume, and their fluorescence signal is detected as a burst . Properties of the molecule can then be determined from the properties of the burst. For this purpose, single molecule fluorescence spectroscopy can be combined with various other methods:
- Förster resonance energy transfer (then often referred to as single molecule FRET or smFRET ) makes it possible to measure distances within a molecule and their changes.
- Polarization- resolved detection: This allows e.g. B. to determine the fluorescence anisotropy of a molecule and thus statements about its rotational diffusion .
- Fluorescence correlation spectroscopy can also be applied to single bursts. This method allows diffusion constants and reaction kinetics to be measured.
- Fluorescence lifetime measurements help separate different molecular species.
- Magnetic and optical tweezers can be used to catch individual molecules and exert forces on them.
As an extension, methods of microfluidics allow the conditions during a measurement (temperature, buffer, chemistry) to be easily influenced and controlled. Furthermore, the residence time of individual molecules in the focus can be extended by increasing the sample viscosity or by immobilizing the molecules on surfaces (see next section). The inclusion of single molecules in (immobilized) vesicles has already been shown.
Measurements on immobilized molecules
In contrast to the measurements described so far, molecules can also be firmly bound to surfaces. These are then z. B. examined with a TIRF microscope over long periods of time. So z. B. the photophysics (blinking, bleaching ) of dyes or the dynamics of individual molecules measured by means of FRET.
Single Particle Tracking (SPT)
The SPT technique (also called single particle tracking ) can also roughly be counted to the class of single molecule fluorescence spectroscopy. Here individual, fluorescence-marked particles ( quantum dots , marked proteins, ...) are tracked over longer periods with the aid of a microscope. From the trajectories measured in the process, conclusions can be drawn about the diffusion properties of the particles and the structure of their surroundings.
literature
- C. Gell, A. Smith, and D. Brockwell: Handbook of Single Molecule Fluorescence Spectroscopy. Oxford Univ. Press 2006, ISBN 978-0-19-852942-2
- Chirlmin Joo, Hamza Balci, Yuji Ishitsuka, Chittanon Buranachai, Taekjip Ha: Advances in Single-Molecule Fluorescence Methods for Molecular Biology . In: Annual Review of Biochemistry . tape 77 , no. 1 , June 2008, ISSN 0066-4154 , p. 51-76 , doi : 10.1146 / annurev.biochem.77.070606.101543 .
Web links
Individual evidence
- ↑ T. Ha, T. Enderle, DF Ogletree, DS Chemla, PR Selvin, S. Weiss: Probing the interaction between two single molecules: fluorescence resonance energy transfer between a single donor and a single acceptor. In: Proceedings of the National Academy of Sciences . tape 93 , no. 13 , June 25, 1996, ISSN 0027-8424 , pp. 6264-6268 , doi : 10.1073 / pnas.93.13.6264 .
- ↑ C. Eggeling, S. Berger, L. Brand, JR Fries, J. Schaffer, A. Volkmer, CA Seidel: Data registration and selective single-molecule analysis using multi-parameter fluorescence detection. In: Journal of Biotechnology . Volume 86, Number 3, April 2001, pp. 163-180, ISSN 0168-1656 . PMID 11257530 .
- ↑ Ted A. Laurence, Youngeun Kwon, Eric Yin, Christopher W. Hollars, Julio A. Camarero, Daniel Barsky: Correlation Spectroscopy of Minor Fluorescent Species: Signal Purification and Distribution Analysis . In: Biophysical Journal . tape 92 , no. 6 , March 2007, ISSN 0006-3495 , p. 2184-2198 , doi : 10.1529 / biophysj.106.093591 .
- ^ Stanislav Kalinin, Alessandro Valeri, Matthew Antonik, Suren Felekyan, Claus AM Seidel: Detection of Structural Dynamics by FRET: A Photon Distribution and Fluorescence Lifetime Analysis of Systems with Multiple States . In: Journal of Physical Chemistry B . tape 114 , no. 23 , June 17, 2010, ISSN 1520-6106 , p. 7983-7995 , doi : 10.1021 / jp102156t .
- ↑ I. Rasnik: Unraveling mechanisms helicase one molecule at a time . In: Nucleic Acids Research . tape 34 , no. 15 , 25 August 2006, ISSN 0305-1048 , p. 4225-4231 , doi : 10.1093 / nar / gkl452 .
- ↑ Bin Wang, Joseph Ho, Jingyi Fei, Ruben L. Gonzalez Jr., Qiao Lin: A microfluidic approach for investigating the temperature dependence of biomolecular activity with single-molecule resolution . In: Lab on a Chip . tape 11 , no. 2 , 2011, ISSN 1473-0197 , p. 274 , doi : 10.1039 / c0lc00157k .
- ↑ I. Cisse, B. Okumus, C. Joo, T. Ha: Single-molecule Chemistry and Biology Special Feature: Fueling protein DNA interactions inside porous nano containers . In: Proceedings of the National Academy of Sciences . tape 104 , no. 31 , May 11, 2007, ISSN 0027-8424 , p. 12646-12650 , doi : 10.1073 / pnas.0610673104 .
- ↑ B. Lounis, J. Deich, FI Rosell, Steven G. Boxer, WE Moerner : Photophysics of Red, a Red Fluorescent Protein, from the Ensemble to the Single-Molecule Level . In: Journal of Physical Chemistry B . tape 105 , no. May 21 , 2001, ISSN 1520-6106 , p. 5048-5054 , doi : 10.1021 / jp010116x .
- ↑ JA Lamboy, H. Kim, KS Lee, T. Ha, EA Komives: Visualization of the nanospring dynamics of the IB ankyrin repeat domain in real time . In: Proceedings of the National Academy of Sciences . tape 108 , no. 25 , June 21, 2011, ISSN 0027-8424 , p. 10178-10183 , doi : 10.1073 / pnas.1102226108 .