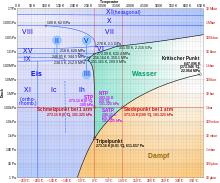
Ice cream I c
Eis I c (spelling also: Eis-I c ) is a metastable crystalline modification of Eis I, which was discovered by Hans König in 1942 and described as a "cubic ice modification". The belief that the crystal structure of Eis I c is a cubic crystal system has largely been retained until today. The oxygen atoms in ice I c are then arranged in the diamond structure. Ice I c is very similar to the ordinary hexagonal ice I h ; at 0.93 g / cm³ it has only a slightly higher density. It is created when it is rapidly cooled to a temperature of 130 to 220 Kelvin (−143 to −53 ° C) and can be heated up to 240 K (−33 ° C) until it turns into the ordinary ice I h .
In the laboratory, ice I c can be produced from supercooled water as well as from amorphous ice and from the high-pressure modifications of ice (ice II, ice III and ice V). It can also form in the upper atmosphere of the earth and it is assumed that it is responsible for the rare 28 ° halo , although there is also a different explanation for this.
Whether the crystal structure of the ice modification "I c " is actually cubic has recently been questioned, because the differences to ice I h are only small: Some authors now call it ice I with stacking faults (stacking-disordered ice, ice I sd ) as a transition structure between the structure of ice I h and the “hypothetical” shape I c , while others see ice I with a stacking fault as a transition phase between I c and I h .
Web links
- Physics of ice (iktp.tu-dresden.de)
- Ice phases (www.idc-online.com)
- Cubic Ice (Ice Ic and Ice XIc) (www.lsbu.ac.uk/water/ice1c.html)
Individual evidence
- ↑ H. König: A cubic ice modification . In: Z. Kristallogr. . 105, No. 1, 1943, pp. 279-286. doi : 10.1524 / zkri.1943.105.1.279 .
- ↑ Ice phases
- ↑ LG Dowell, AP Rinfre: Low-temperature forms of ice as of studied by x-ray diffraction . In: Nature . 189, No. 4757, 1960, pp. 1144-1148. bibcode : 1960Natur.188.1144D . doi : 10.1038 / 1881144a0 .
- ^ BJ Murray, AK Bertram: Formation and stability of cubic ice in water droplets . In: Phys. Chem. Chem. Phys. . 8, No. 1, 2006, pp. 186-192. bibcode : 2006PCCP .... 8..186M . doi : 10.1039 / b513480c . PMID 16482260 .
- ^ BJ Murray: The Enhanced formation of cubic ice in aqueous organic acid droplets . In: Env. Res. Lett. . 3, No. 2, 2008, p. 025008. bibcode : 2008ERL ..... 3b5008M . doi : 10.1088 / 1748-9326 / 3/2/025008 .
- ↑ E. Mayer, A. Brucker Hall: Cubic ice from liquid water . In: Nature . 325, No. 12, 1987, pp. 601-602. bibcode : 1987Natur.325..601M . doi : 10.1038 / 325601a0 .
- ↑ LG Dowell, AP Rinfre: Low-temperature forms of ice as of studied by x-ray diffraction . In: Nature . 189, No. 4757, 1960, pp. 1144-1148. bibcode : 1960Natur.188.1144D . doi : 10.1038 / 1881144a0 .
- ^ JE Bertie, LD Calvert, E. Whalley: Transformations of Ice II, Ice III, and Ice V at Atmospheric Pressure . In: J. Chem. Phys. . 38, No. 4, 1963, pp. 840-846. bibcode : 1963JChPh..38..840B . doi : 10.1063 / 1.1733772 .
- ^ BJ Murray: The formation of cubic ice under conditions relevant to Earth's atmosphere . In: Nature . 434, No. 7030, 2005, pp. 202-205. bibcode : 2005Natur.434..202M . doi : 10.1038 / nature03403 . PMID 15758996 . , [1]
- ^ Color and Light in Nature
- ^ E. Whalley: Scheiner's Halo: Evidence for Ice I c in the Atmosphere . In: Science . 211, No. 4480, 1981, pp. 389-390. bibcode : 1981Sci ... 211..389W . doi : 10.1126 / science.211.4480.389 . PMID 17748273 .
- ↑ Scheiner's Halo: Cubic Ice or Polycrystalline Hexagonal Ice?
- ↑ TRIGONAL ICE CRYSTALS IN EARTH'S ATMOSPHERE journals.ametsoc.org, September 2015
- ↑ Stacking disordered ice; Ice Isd
- ↑ Stacking disorder in ice I pubs.rsc.org, 2015
- ↑ Structure and transformations of ice phases in the presence of the gases helium, neon and argon (pdf direct download)