Extractive rectification
The extractive rectification is a method in chemical process engineering for separating azeotropic or close-boiling mixtures. These mixtures with low relative volatility of the components involved can either not be separated at all by normal rectification (azeotropes) or only with disproportionate effort.
Procedure
Extractive rectification uses an additive, the so-called entrainer , which selectively increases the volatility of one of the components or changes the activity coefficients of the substances to be separated significantly in different directions and thus the separation factor
γ: activity coefficient
P s : vapor pressure of the pure substance
changed and as far away from 1 (= azeotrope) as possible. In addition, the entrainer should not introduce a new azeotrope, i.e. not form an azeotrope with any of the substances to be separated, and it should have a higher boiling point or a significantly lower vapor pressure than the substances to be separated. The additive can thus be drawn off via the bottom of the column together with one of the substances to be separated and separated (regenerated) from this in a second column. The entrainer is then fed back into the extraction column.
example
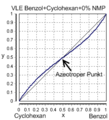
A typical example of an extractive rectification is the separation of the azeotropic mixture of benzene and cyclohexane . By adding significant amounts of an additive (in the example up to 40 mol percent N- methyl-2-pyrrolidone ) the azeotrope is canceled (liquid composition x = vapor composition y) and separation is made possible.
Construction of a technical system
Usually, the entrainer, which is usually a high boiler, is fed in close to the top of the column and the mixture to be separated is fed into a lower tray in order to achieve the best possible mixing.
Another basic requirement for the choice of a suitable entrainer for extractive rectification is complete miscibility with all components of the mixture to be separated. The substance should also be inexpensive, available, non-toxic and have a low viscosity .
Entrainer selection
A suitable substance can be selected with the help of fact databases for vapor-liquid equilibria and activity coefficients (especially activity coefficients at infinite dilution, γ ∞ ). Ionic liquids can also be used as entrainers. For the selection of the entrainer - tailored to the separation problem - models for estimating activity coefficients, such as UNIFAC , are used.
Individual evidence
- ^ Brockhaus ABC Chemie , VEB FA Brockhaus Verlag Leipzig 1965, p. 387.