Fumed silicon dioxide
Fumed silica (historic, but technically incorrect, and fumed silica , English fumed silica ) is a synthetic, colloidal materials with defined properties and particle size, which as a filler in plastics is used. It consists entirely of amorphous silicon dioxide particles (SiO 2 ) that have aggregated into larger units . The same applies to precipitated silicon dioxide (historically: precipitated silica), but this differs in its production method and properties.
history
The production of fumed silicon dioxide was started at Degussa in 1944 using a process developed by Harry Kloepfer .
Manufacturing method and properties
The processes used to make precipitated and fumed silica are compared in the following figure:
Sand (crystalline silicon dioxide) is used as the starting material for both precipitated and pyrogenic silicon dioxide.
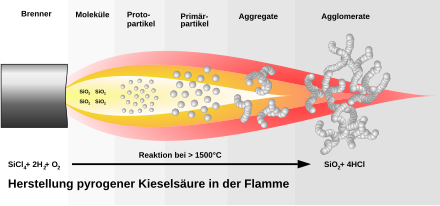
To produce fumed silicon dioxide, the sand is first reduced with carbon and the silicon produced is then reacted with chlorine to form silicon tetrachloride . The last step is the high-temperature pyrolysis of the silicon tetrachloride with oxyhydrogen (H 2 + O 2 ). A homogeneous mixture of vaporous silicon tetrachloride, hydrogen, oxygen and an inert gas is burned with a burner in a cooled combustion chamber. Hydrochloric acid is formed as a by-product . First, droplet-like silicon dioxide particles are formed in the flame, which attach to one another in a chain-like manner and thus form three-dimensional secondary particles via branches. These in turn accumulate to form tertiary particles. This is shown in the next figure:
The result is a white powder with an extremely low ( bulk ) density and high surface . When it is used as a filler, its three-dimensional structure makes it strongly thickening, thixotropic and protects against sedimentation. It is assumed that the mobility of polymer chains is reduced by strong adsorption on the silicon dioxide particles. The thixotropy is therefore caused by hydrogen bonds between primary particles, which, however, are temporarily destroyed by shear. The particles have a diameter of 5 to 50 nm, a surface area of 50 to 600 m 2 / g, a density of 160 to 190 kg / m³ and are not porous.
Important manufacturers of synthetic silicon dioxide are Evonik Industries (formerly Degussa, trade name: Aerosil ), Wacker Chemie (HDK), Rhodia and WR Grace .
Classification
The different variants of silicon dioxide (all SiO 2 ) can be divided into natural, crystalline silicon dioxide, such as quartz powder ; Natural, amorphous silicon dioxide, such as kieselguhr (= diatomaceous earth) and synthetic, amorphous silicon dioxide such as precipitated (CAS no .: 112926-00-8) and pyrogenic (CAS no .: 112945-52-5 and 60842-32-2) Silicon dioxide.
Fillers containing quartz are characterized by their special hardness and chemical resistance (quartz content at least 95% according to DIN 55926). Natural, amorphous silicon dioxide is used as an inexpensive way to improve mattness, drying and sandability in paints. Pyrogenic silicas are mostly used as a thixotropic agent and not as a filler.
properties
The following table compares the properties of fumed and precipitated silica.
| property | Fumed silicon dioxide | Precipitated silica |
|---|---|---|
| Specific BET surface area in m 2 · g −1 | 50-600 | 30-800 |
| Primary particle size in nm | 5-50 | 5-100 |
| Aggregate or agglomerate size in µm | 0.1-100 | 1-40 |
| Density in g cm −3 | 2.2 | 1.9-2.1 |
| Tamped volume in ml / 100 g | 1000-2000 | 200-2000 |
| Loss on drying in% | ≤ 2.5 | 3-7 |
| Loss on ignition in% | 1-3 | 3-7 |
| PH value | 3.6-4.3 | 5-9 |
| Pore diameter in nm | non-porous up to approx. 300 | > 30 |
Important differences between precipitated silicon dioxide and pyrogenic silicon dioxide exist in the size of the particles and the purity: Precipitated silica is in the µm range, while fumed silica is in the nm range. In addition, precipitated silica consists of 93% SiO 2 and therefore contains more impurities than pyrogenic silica (99% SiO 2 ).
Health hazards
Silicon dioxide (dissolved as silica ) is a constituent part of every human being (about 1 g). Since silicon dioxide (in very small amounts) promotes growth, it appears to have a biological function. Silica is only toxic to humans in high concentrations (≥ 100 mg / kg). Solid silicon dioxide that is absorbed can dissolve and be excreted as silica.
Unlike dusts from minerals such as quartz and cristobalite , there is no suspicion of causing lung diseases ( silicosis ) for the amorphous products . For pyrogenic and wet-precipitated silicon dioxide, a limit value for the inhalable dust fraction ( workplace limit value ) of 4 mg / m³ was set.
Overview literature
- Evonik , “AEROSIL® - Pyrogenic Silica”, 8th edition
Individual evidence
- ↑ Coating materials: terms from DIN standards . 1st edition. Vincentz [and a.], Hannover 2001, ISBN 3-87870-721-5 , pp. 157 ( limited preview in Google Book search).
- ↑ Mechtild Wolf (ed.): Always an idea better: Researcher and inventor at Degussa . Degussa AG, Frankfurt am Main 1998, pp. 76-93.
- ↑ PR Garrett: Defoaming. Theory and Industrial applications . CRC Press, USA 1992, ISBN 0-8247-8770-6 , pp. 239-240 .
- ↑ Vollrath Hopp: Fundamentals of chemical technology: for study and professional training . Wiley-VCH, Weinheim u. a. 2001, ISBN 3-527-29998-X , p. 312.
- ↑ Otto W. Flörke u. a .: silica. In: Ullmann's Encyclopedia of Industrial Chemistry. 2008, Weinheim: Wiley-VCH, doi : 10.1002 / 14356007.a23_583.pub3 .
- ↑ Detlef Gysau: Fillers: Basics and Applications . 2., revised. Edition. Vincentz Network, Hannover 2005, ISBN 3-87870-337-6 , p. 131 ( limited preview in Google Book search).
- ↑ Thomas Brock, Michael Groteklaes, Peter Mischke: Textbook of paint technology . 2nd Edition. Vincentz Network, Hannover 2000, ISBN 3-87870-569-7 , p. 161 ( limited preview in Google Book search).
- ↑ Hans-Frieder Eberhardt: How is a vacuum insulation panel created? (Lecture: VIP building: 2nd symposium “Experiences from practice” . June 16/17, 2005, Wismar).
- ^ Anten Chemical Co., Ltd .: Fumed silica and Precipitated silica-Anten Chemical Co., Ltd. Retrieved October 15, 2018 .
- ^ Hans-Dieter Belitz , Werner Grosch , Peter Schieberle : Textbook of food chemistry . 6th completely revised edition. Springer, Berlin / Heidelberg 2008, ISBN 978-3-540-73201-3 , p. 438 , doi : 10.1007 / 978-3-540-73202-0 .
- ↑ Entry on silicon dioxide, colloidal in the GESTIS substance database of the IFA , accessed on October 8, 2016(JavaScript required) .