F-53
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
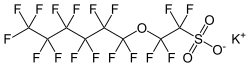
|
|||||||
| General | |||||||
| Surname | F-53 | ||||||
| other names |
Potassium 2 - [(1,1,2,2,3,3,4,4,5,5,6,6,6-tridecafluorohexyl) oxy] -1,1,2,2-tetrafluoroethanesulfonate |
||||||
| Molecular formula | C 8 F 17 KO 4 S | ||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 554.22 g mol −1 | ||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
F-53 is the potassium salt of a perfluorosulfonic acid and belongs to the group of perfluorinated and polyfluorinated alkyl compounds (PFAS).
use
F-53 has been used as an antifoggant (mist suppressor) in Chinese electroplating technology for chrome plating since the 1970s . The amount used was estimated at 20 to 30 tons in 2009. Compared to F-53B , in which a fluorine atom is substituted by a chlorine atom, there are price disadvantages in the synthesis .
Hazard and risk assessment
F-53 has a similarly strong binding affinity for liver fatty acid binding protein as PFOS, which is bioaccumulative and was included in the Stockholm Convention .
In water treatment, it behaves similar to perfluorooctanesulfonic acid (PFOS).
See also
- Perfluorooctanoic acid (PFOA)
Individual evidence
- ↑ Zhanyun Wang, Ian T. Cousins et al. a .: Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors. In: Environment International . 60, 2013, pp. 242-248, doi : 10.1016 / j.envint.2013.08.021 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b c Siwen Wang, Jun Huang, Yang Yang, Yamei Hui, Yuxi Ge, Thorjørn Larssen, Gang Yu, Shubo Deng, Bin Wang, Christopher Harman: First Report of a Chinese PFOS Alternative Overlooked for 30 Years: Its Toxicity, Persistence , and Presence in the Environment. In: Environmental Science & Technology . 47 (18), 2013, pp. 10163-10170, doi : 10.1021 / es401525n .
- ↑ Weixiao Cheng, Carla A. Ng: Predicting Relative Protein Affinity of Novel per- and polyfluoroalkyl Substances (PFASs) by An Efficient Molecular Dynamics Approach. In: Environmental Science & Technology. 52 (14), 2018, pp. 7972-7980, doi : 10.1021 / acs.est.8b01268 .