Fmoc protecting group
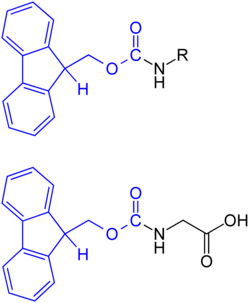
Fluorenylmethoxycarbonyl ( Fmoc ) is a protecting group that is used to protect amines . One area of application is the synthesis of peptides . With this protective group it is possible to protect the next amino acid in the chain growth in the direction of the N -terminal end of the peptide. The combination of N -Fmoc and tert -butyl esters or its chemically related polymer-bound analogues is an ideal orthogonal protective group combination and is widely used in solid-phase peptide synthesis.
meaning
The fluorenylmethoxycarbonyl group forms a central element in the strategy of solid-phase peptide synthesis. Ideally, in an automatable synthesis, such as the one mentioned, it should be possible to remove all necessary protective groups separately and undisturbed from one another. This concept is called orthogonality of the protecting groups.
In peptide synthesis, one usually has to deal with three groups of protective groups: First, the protective group in the direction of the future N- terminal end, for example Fmoc. Furthermore a protective group for the C -terminal end; In solid-phase peptide synthesis, this is formed by a resin to which the first amino acid is bound. Premature detachment of the growing peptide would reduce the yields considerably. The third type are the protective groups for any interfering functional groups in the amino acids that are to be incorporated into the peptide.
Here, Fmoc is an exception among the protective groups due to its extraordinary property of being stable in acidic conditions, but unstable in basic conditions. Most of the other protecting groups are split off under acidic conditions.
In solid-phase peptide synthesis, peptides on resins according to Merrifield and further developments of this technology are produced automatically with the help of synthesis robots. The standard protocols used enable peptide coupling in yields of over 99.99%, which is necessary in order to be able to synthesize and later purify peptides in the 10 kDa range.
Deprotect
The protective group can be removed again under weakly basic conditions, but is stable under acidic conditions. It can therefore be used well in conjunction with the so-called Boc protective group ( tert -butyloxycarbonyl) and the protective group for carboxylic acids commonly used in this context , the tert -butyl ester, and is complementary to this because the Boc and also the tert-butyl ester Group acidic (usually with concentrated trifluoroacetic acid ) is removed. When Fmoc is deprotected, the acidic hydrogen atom is removed with the aid of a base (usually a secondary amine). In a mechanism called E1 cb , the protective group system collapses to a quasi- aromatic system and the free amine (e.g. ester of a peptide ), as shown in the reaction mechanism:
The proton in the 9-position of the fluorenyl ring (pK a = 22.6 in dimethyl sulfoxide ) can be removed under relatively mild conditions. This makes it possible for the deprotection with 20% piperidine , a relatively weak base, in dimethylformamide (DMF) to take place within a few minutes (30 min). On the basis of characteristic UV absorption at 295 nm (ε = 7800) one can follow the correct course of the deprotection and thereby identify problematic peptide couplings in peptide syntheses using a synthesizer based on the slow or poor cleavage of the protective group.
Protection of the amino function
In the absence of other interfering groups on the part of the amino acid, the amino function is normally protected by reaction with fluorenylmethoxycarbonyl chloride (Fmoc-Cl) or N - (9-fluorenylmethoxycarbonyloxy) succinimide (Fmoc-OSu) in the presence of an aqueous sodium hydrogen carbonate solution with the appropriate Amino acid. The Fmoc derivatives of all common amino acids are commercially available.
Alternatively, the amino function of an amino acid can also be protected with the Fmoc group if the N -hydroxysuccinimide derivative Fmoc-ONSu is used instead of fluorenylmethoxycarbonyl chloride .
literature
W. Chan (Author), WC Chan (Editor), Peter D. White. In: Fmoc Solid Phase Peptide Synthesis: A Practical Approach (Paperback) , Oxford University Press Oxford New York 2000, ISBN 0-19-963724-5 .
Individual evidence
- ^ A b Louis A. Carpino: The 9-Fluorenylmethoxycarbonyl Family of Base-Sensitive Amino-Protecting Groups. In: Accounts of Chemical Research 20 ( 1987 ) pp. 401-407.
- ↑ Paul BW th Kortenaar, Benno G. van Dijk, Marjolijn Peeters, Bert J. Raaben, PJ Hans M. Adams and Godefridus I. Tesser: Rapid and efficient method for the preparation of Fmoc-amino acods starting from 9-fluorenyl . In: Int. J. Peptide Protein Res. Vol. 27, 1986, pp. 398-400.
