Friedlaender quinoline synthesis
The Friedlaender quinoline synthesis , also called Friedlaender condensation , is a name reaction in organic chemistry . It is mainly used to manufacture quinoline and is named after the German chemist Paul Friedlaender (1857–1923).
Overview reaction
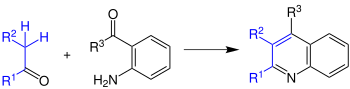
In the Friedlaender quinoline synthesis, aromatic aldehydes or ketones react with aldehydes or ketones to form quinols.
The radicals R 1 -R 3 can be both hydrogen atoms and organyl radicals . The synthesis is catalyzed by acids or bases . Whereby acids probably work better. In addition, the reaction must always be performed in solvents as they otherwise under severe conditions such as temperatures of up to 200 ° C must be carried out. Upon closer inspection, it was found that there are two distinct steps in which the reaction occurs:
1. Condensation of the amino group with the carbonyl group
2. Intramolecular aldol condensation .
Both steps take place with elimination of water .
mechanism
The variant presented here is the base-catalyzed reaction. There is also an acid-catalyzed reaction. The Pfitzinger reaction and the Niementowski quinoline synthesis are based on the concept of the Friedlaender quinoline synthesis mechanism presented here:
First, the carbonyl compound 1 is mixed with a base (B). This removes a proton from the aldehyde or ketone in the α position to the carbonyl group . This forms an enolate 2 . With the electrons of the double bond that has formed, this molecule attacks the carbon atom of the carbonyl group on the aromatic six-membered ring 3 , as a result of which the carbonyl group of molecule 3 changes into an alcoholate 4 . This alcoholate removes a proton from the protonated base from step 2 and thus becomes an alcohol . By splitting off water , the α, β-unsaturated carbonyl compound 6 is then formed from 5 . Now at 6 an intramolecular attack of the amino group takes place on the remaining carbonyl group, whereby a second six-membered ring is formed in the molecule 7 . Thus, molecule 7 is a bicyclic compound. The amino group and the alcoholate of the newly formed heterocyclic six-membered ring carry out a proton transfer. The hydroxyl group formed in this way from an alcoholate now attacks the hydrogen atom on the nitrogen atom of 8 , as a result of which this hydroxyl group is also deposited in the form of water. Quinoline derivative 9 is thus formed .
application
The Friedlaender quinoline synthesis is used to produce quinolines, naphthyridines and other polycyclic heterocyclic derivatives - mostly in high yields.
Individual evidence
- ↑ Winfried R. Pötsch u. a .: Lexicon of important chemists . Bibliographisches Institut, Leipzig 1998, ISBN 3-323-00185-0 , pp. 157-158.
- ↑ a b c Zerong Wang: Comprehensive Organic Name Reactions and Reagents . John Wiley & Sons, New Jersey 2009, ISBN 978-0-471-70450-8 , pp. 1137-1141 .
- ↑ after: BP Mundy, MG Ellerd, FG Favaloro: Name Reactions and Reagents in Organic Synthesis . 2nd edition, Wiley-Interscience, Hoboken, NJ 2005, ISBN 978-0-471-22854-7 , p. 258.