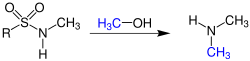Fukuyama amine synthesis
The Fukuyama amine synthesis , also known as the Fukuyama-Mitsunobu reaction , is a name reaction in organic chemistry . Tohru Fukuyama and his co-workers discovered this synthesis in 1995. It is a two-step synthesis of secondary amines from primary amines. By using the reagents triphenylphosphine and diethylazodicarboxylate (DEAD) or diisopropylazodicarboxylate (DIAD) it shows a certain similarity to the Mitsunobu reaction .
Overview reaction
The Fukuyama amine synthesis is a reaction with protecting groups . The primary amine is usually protected as a sulfonamide by reaction with o -nitrobenzenesulfonic acid chloride . For the sake of clarity, the protective group is only briefly outlined below:
The reaction needs a neutral environment. In addition, an alcohol must be added. This and the primary starting material then result in the secondary product . The reaction is catalyzed by the presence of triphenylphosphine and diethylazodicarboxylate (DEAD) or diisopropylazodicarboxylate (DIAD), whereby the use of DIAD makes the reaction a lot more efficient. In order to detach the product from the protective group again, a mixture of thiophenol and potassium carbonate is added.
mechanism
The following reaction mechanism has been proposed:
First the protective group is formed. To do this, the o -nitrobenzenesulfonic chloride (2) reacts with the primary amine (1) . After a few rearrangements, hydrogen chloride is split off. Now a mixture of triphenylphosphine and DEAD is used and deprotonates the nitrogen atom of protective group 3 . An intermediate product 4 is formed . In the next step, the protonated triphenylposphane DEAD reacts with the added alcohol 5 to form another intermediate 6 . The two intermediates 4 and 6 now react with each other, so that the nitrogen atom now two alkyl radicals having 7 . Now the above-mentioned mixture of thiophenol and potassium carbonate is added. The thiophenolate binds to the sulfur atom from the protective group. The nitrogen atom deprotonates the hydrogen carbonate and thus splits off from the protective group, the secondary amine (8) being formed.
application
The Fukuyama reaction is used for the synthesis of secondary and tertiary amines. In addition, polyamides can also be produced. It is particularly suitable for solid phase syntheses. The synthesis of N-methylamino acids from aliphatic and aromatic amino acids such as alanine , valine or tryptophan works particularly well . Very high yields and high purity are achieved here.
criticism
The Fukuyama synthesis is a purely laboratory process. Despite the high yields, the atom economy of the Fukuyama synthesis is so bad , because of the formation of stoichiometric amounts of several waste materials , that no industrial synthesis for secondary amines based on this reaction can be realized.
Individual evidence
- ↑ a b c d Zerong Wang: Comprehensive Organic Name Reactions and Reagents . John Wiley & Sons, New Jersey 2009, ISBN 978-0-471-70450-8 , pp. 1159-1163 .
- ↑ a b c d e Tohru Fukuyama, Chung-Kuang Jow, Mul Cheung: 2- and 4-Nitrobenzenesulfonamides: Exceptionally Versatile Means for Preparation of Secondary Amines and Protection of Amines. In: Tetrahedron Letters . tape 36 , 1995, pp. 6373-6374 , doi : 10.1021 / ol100914b .
- ↑ Xiaodong Lin, Hilary Dorr, John M. Nuss: Utilization of Fukuyama's sulfonamide protecting group for the synthesis of N-substituted α-amino acids and derivatives . In: Tetrahedron Letters . tape 41 , no. 18 , April 29, 2000, pp. 3309-3313 , doi : 10.1016 / S0040-4039 (00) 00424-X .
- ↑ Yosup Rew, Murray Goodman: Solid-Phase Synthesis of Amine-Bridged Cyclic enkephalin Analogues via on-resin cyclization Utilizing the Fukuyama - Mitsunobu reaction . In: The Journal of Organic Chemistry . tape 67 , no. 25 , December 2002, p. 8820-8826 , doi : 10.1021 / jo020447l .
- ↑ a b Lihu Yang, Kuenley Chiu: Solid phase synthesis of Fmoc N-methyl amino acids: Application of the Fukuyama amine synthesis . In: Tetrahedron Letters . tape 38 , no. 42 , October 20, 1997, pp. 7307-7310 , doi : 10.1016 / S0040-4039 (97) 01774-7 .