Green leaf fragrances
| Green leaf fragrances | ||
| Systematic name ( IUPAC ) | Common name | Structural formula |
| 1-hexanol | Capro alcohol |
|
| Hexanal | Capronaldehyde |

|
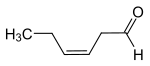
| ( Z ) -hex-3-enal | Leaf aldehyde cis -3-hexenal |
|
| ( E ) -hex-2-enal | trans -2-hexenal |

|
| ( E ) -hex-2-en-1-ol | trans -2-hexen-1-ol |
|
| ( Z ) -Hex-3-en-1-ol | Leaf alcohol cis -3-hexen-1-ol |

|
| ( Z ) -Hex-3-en-1-ylacetate | Acetic acid ester of the leaf alcohol cis -3-hexen-1-ol |

|
Green leaf fragrances ( English green leaf volatiles - GLV) is an umbrella term for the volatile leaf fragrances that are released when plant parts are mechanically damaged and that act as phytoalexins for the plant.
These are C 6 bodies from the substance groups alcohols and aldehydes . Occasionally, derivatives of these compounds, such as the acetic acid esters of alcohols, are also counted among the green leaf fragrances. The quantitative distribution of the individual components varies greatly between different plants. Many GLVs are biosynthesized from α-linolenic acid , which is cleaved by lipoxygenases via a peroxycarboxylic acid to form volatile ( Z ) -3-hexenal and a non-volatile C 12 compound. The 3-hexenal is reduced to the most famous representative of the green leaf fragrances , the cis -3-hexenol , which is also known as leaf alcohol . This is mostly converted to cis -hexenyl acetate , the acetic acid ester of leaf alcohol.
The green leaf fragrances have their function in killing fungi ( fungicide ) and bacteria ( bactericide ) as well as deterring other predators ( herbivores ), mainly insects . These properties are known as direct defense . In addition, current research in chemical ecology has now shown that they can also attract representatives of the 3rd trophic level , i. H. Predators ( predators ) or parasitoids (z. B. parasitic wasps ), which feed on the herbivores and therefore the plant come to the rescue.
Individual evidence
- ↑ LM Schoonhoven, JJA van Loon, Marcel Dicke: Insect-plant biology. Oxford University Press 2005, ISBN 0-19-852595-8 ( limited preview in Google Book Search), p. 60.
- ↑ Stefan Schwab: Leaf fragrance samples of various host tree species of the horse chestnut leaf miner (Cameraria ohridella DESCHKA & DIMIĆ 1986). (PDF; 10.8 MB) Dissertation at the Friedrich-Alexander-University Erlangen-Nürnberg , 2008.
- ↑ a b John D'Auria: The world of natural substances: Investigations of ester compounds and acyltransferases on the model system Arabidopsis thaliana. (PDF; 9.9 MB) Max Planck Institute for Chemical Ecology , Jena, Activity Report 2008.