Hemiamyloidity
In mycology, hemiamyloidiness refers to a special case of cell wall amyloidy, in which the blue coloration by means of iodine reagents only occurs after pretreatment with potassium hydroxide solution , whereas when iodine Lugol's solution is applied directly, a red reaction occurs, whereas Melzer’s reagent does not cause any reaction. Hemi-amyloidity is only known in the case of tubular fungi , but it is widespread here and an important taxonomic differentiation criterion. If cell walls stain blue with iodine reagents even without pretreatment with potassium hydroxide, this is called euamyloidy; the umbrella term for both variants is amyloidy .
properties
When iodine reagents are added to the water preparation, a hemiamyloid cell wall structure does not immediately react blue, but only after a treatment with potassium hydroxide (KOH). Without KOH treatment, the result depends very much on the type of iodine reagent: With Lugol's solution (IKI), hemiamyloid structures react red to red-brown; this red reaction is completely suppressed when using Melzer's reagent (MLZ) due to the high chloral hydrate concentration (apparent inamyloidy). The alternative to hemiamyloid is called euamyloid. Euamyloid as well as KOH-pretreated hemiamyloid structures react blue regardless of the type of iodine reagent. Hemiamyloid and euamyloid reactions often occur mixed, either at spatially separate locations on the ascus (e.g. apical ring euamyloid, lateral wall hemiamyloid), or mixed in the same wall region. In the latter case, an overlay of blue and red can be observed in Lugol's solution without KOH pretreatment. However, since the euamyloid reaction starts at a lower iodine concentration than the hemiamyloid, in the case of the mixed type there is a color change from blue to (more or less dirty) red-brown or, in the case of asci with a completely reactive cell wall, to rainbow-like colors, while the iodine reagent diffuses into the water preparation.
| inamyloid | hemiamyloid | euamyloid | ||||
| IKI | MLZ | IKI | MLZ | IKI | MLZ | |
| before KOH | - | - | red | - | blue | blue |
| after KOH | - | - | blue | blue | blue | blue |
Hemiamyloid (red) IKI reaction without KOH pretreatment compared to euamyloid (blue) and inamyloid (negative). Only the hemiamyloid reaction depends on the iodine reagent used (IKI, MLZ) and the pretreatment with KOH: Hemiamyloid structures show no reaction in MLZ, but react blue if KOH-pretreated, regardless of the iodine reagent.
| IKI (= Lugol's solution) | MLZ (= Melzer's reagent) | |
| before KOH |
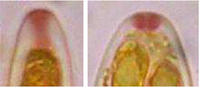
|

|
| after KOH |

|

|
Iodine reaction of hemiamyloid apical rings of the tubes of Hysteropezizella (Helotiales) depending on iodine reagent (IKI, MLZ) and pretreatment with KOH.
Occurrence, meaning
Hemi-amyloidity occurs in many groups of ashlar fungi . The Lecanorales and most of the Ostropales (many representatives belong to the lichens that live symbiotically with algae ) have a hemiamyloid outer ascus wall layer. About 20% of the Helotiales have hemiamyloid apical rings compared to an estimated 50% with euamyloid apical rings. In cup-like Ling with operculaten Asci and Pyrenomycetes hemiamyloide reactions, however, are rare. Although the hemiamyloidity is a very valuable taxonomic characteristic that allows to differentiate between species and genera, this type of reaction, especially the red reaction in Lugol's solution, is often overlooked to this day. This happens especially because in mycology (but not in lichenology ) Melzer's reagent has wrongly almost completely replaced Lugol's solution, which had been in use up until then, since 1924. Due to the frequency of hemiamyloidity in lichens, the lichenologists did not participate in this change, but continued to use Lugol's solution. The common method of swelling herbarized mushrooms in KOH prior to testing further contributes to the fact that hemiamyloidity is often overlooked.
Chemism
The chemical background of hemiamyloidity is not clear. The carbohydrate chains may be in such a way that short helical sections alternate with short or longer elongated sections. Similar to the dextrinoid nature of glycogen, the short helical sections would cause the red reaction by incorporating the iodine atoms into the spiral, while the elongated sections could curl up under the action of potassium hydroxide, so that long helical sections are created that give a blue color when iodine is stored. The hypothetical spiral structure of these macromolecules seems to have something to do with the elasticity of the ascus walls, which is a prerequisite for the explosive, active ejection of the ascospores with the release of the high cell turgor . This applies in particular to the area of the ascusporus (apical ring) through which the spores are pressed when the ascus bursts.
literature
- Baral, H.-O. (1987): Lugol's solution / IKI versus Melzer's reagent: hemiamyloidity, a universal feature of the ascus wall. Mycotaxon 29: 399-450.
- Baral, H.-O. (2007): On the iodine reaction in ascomycetes. Ink 51 (2)
- Baral, H.-O. (2009): Iodine reaction in Ascomycetes: why is Lugol's solution superior to Melzer's reagent? https://in-vivo-veritas.de/articles/iodine-reaction-in-ascomycetes-why-is-lugols-solution-superior-to-melzers-reagent/
- Blackwell, M., AJ Kinney, PT Radford, CM Dugas, & RL Gilbertson. 1985. The chemical basis of Melzer's reaction. MSA, Gainesville, Florida, August 1985 (abstract)
- Kohn, LM, and RP Korf. 1975. Variation in ascomycete iodine reactions: KOH pretreatment explored. Mycotaxon 3: 165-172.
- Rossman, AY 1980. The iodine reaction: Melzer's vs. IKI. MSA newsletter 31:22.