Homolytic cleavage
In homolytic cleavage or homolytic bond cleavage and radical reaction , a covalent bond between two atoms is split by external influences such as high-frequency light ( photolysis ) or heat ( thermolysis ), so it is a type of dissociation . Here, after the cleavage of A – B, one binding electron remains with each of the previous binding partners (A and B), radicals are formed (A · and B ·): Radicals are formed when particles with less polar bonds react. They occur particularly frequently in the gas state, when the kinetic energy of the particles is large enough to enable bond cleavage on collision.
The formation of radicals takes place preferentially in a non-polar environment, since the formation of ions is often favored in a polar environment. The homolytic cleavage is used for the generation of radicals, which are used as starting radicals
- in polymer chemistry ,
- in the halogenation of alkanes
- in the side chain halogenation of alkylated aromatics ( SSS rule )
- in halogenation in the allyl position of alkenes
to have.
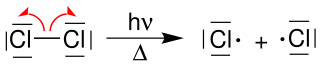
An example of radical generation is the light-induced homolysis of chlorine :
During the photolysis of acetone , a methyl radical and an acetyl radical are initially formed .
The reverse of homolytic cleavage is the recombination of two radicals with the formation of a single bond.
Dissociation energies
Dissociation energies are a measure of the stability of covalent bonds. They indicate the amount of energy that is necessary to split a bond homolytically. Therefore, this value is greater for multiple bonds than for single bonds.
See also
- Heterolytic cleavage (as opposed to homolysis)
Web links
Individual evidence
- ↑ Entry on homolysis. In: Römpp Online . Georg Thieme Verlag, accessed on June 15, 2014.
- ^ MD Lechner, K. Gehrke and EH Nordmeier: Makromolekulare Chemie , 4th edition, Birkhäuser Verlag, 2010, pp. 54–55, ISBN 978-3-7643-8890-4 .
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, p. 205, ISBN 3-342-00280-8 .
- ↑ Entry on methyl .... In: Römpp Online . Georg Thieme Verlag, accessed on June 15, 2014.