Hydroamination
The hydroamination is a reaction in the organic chemistry that with excellent atom economy expires.
The formal addition of a secondary amine to carbon-carbon multiple bonds creates tertiary amines. Alkenes or alkynes which react with the amine can be used as starting materials :
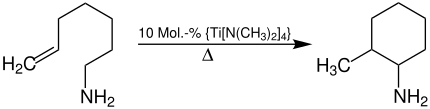
The hydroaminoalkylation of primary amines has so far been limited to the intramolecular titanium-catalyzed ring closure reaction of ω-aminoalkenes:
The hydrohydrazination of alkynes is closely related to hydroamination . In this case is formed by the addition of a hydrazine to an alkylated hydrazone .
Such reactions often proceed through catalysis with transition metal catalysts . The early transition metals of the titanium group have proven to be particularly suitable for this.
Individual evidence
- ^ R. Kubiak, I. Prochnow, S. Doye , Angewandte Chemie 2009, 121, pp. 1173–1176, DOI: 10.1002 / anie.200805169 .
- ↑ I. Prochnow, R. Kubiak, ON Frey, R. Beckhaus , S. Doye, ChemCatChem 2009, 1, pp. 162-172.
- ^ I. Prochnow, P. Zark, T. Müller, S. Doye, Angewandte Chemie 2011, 123, pp. 6525-6529, DOI: 10.1002 / anie.201101239 .
- ↑ C. Cao, Y. Shi, A. Odom, Org. Lett. 2002, 4, 2853-2856.