Isothermal titration calorimetry
Isothermal titration calorimetry (ITC) is a biophysical technique that is used to determine thermodynamic parameters of biochemical binding processes. Usually the binding of small molecules, such as medically active substances, to larger macromolecules ( proteins , DNA , etc.) is examined and thermodynamically characterized. This allows conclusions to be drawn about the energetics of the bond as well as the number and the ratio of the particles involved.
Thermodynamic measurements
The ITC is a quantitative measurement method with which the binding affinity K a (binding strength), binding enthalpy Δ H (binding heat) and the binding stoichiometry n (ratio of the particles involved) of the interaction under investigation can be measured. These measurements allow the calculation of the change in Gibbs' enthalpy Δ G and the entropy Δ S through the following relationship:
(where R is the gas constant and T is the absolute temperature ).
Measuring device
An isothermal titration calorimeter consists of two identical, highly thermally conductive materials ( gold or Hastelloy ) that are built into adiabatically encased cells. Sensitive thermocouples record the temperatures of the liquids within the cells with high accuracy. One of the two cells is used as a reference cell (with water or buffer solution ), the other serves as a sample cell in which the macromolecule is in exactly the same solution (water or buffer). The liquid volume per cell is usually a few ml. Both cells are initially heated with constant power (usually below 1 mW), with a feedback mechanism regulating the heating mechanism of the sample cell depending on the temperature in the cell.
execution
Precisely known amounts of the ligand are added during the experiment, which results in either an uptake or a release of heat to the liquid within the sample cell. The time-dependent supply of energy by the heating mechanism of the sample cell, which is required to maintain the same temperature as in the reference cell, is measured.
In the case of exothermic reactions (i.e. when heat is released during the binding of the ligand by the macromolecules in the solution), the temperature of the solution in the sample cell rises so that less energy has to be supplied to heat the sample cell in exactly the same way as the reference cell. Conversely, in the case of an endothermic reaction that cools the solution in the sample cell, more energy must be supplied. In both cases, the amount of energy supplied is adjusted using the feedback mechanism mentioned above.
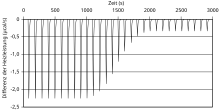
evaluation
The measurement results are plotted as the power required to maintain the same temperatures in μcal / s as a function of the time in s . Also .mu.J (instead μcal) as an energy unit is in use. The diagram of the raw data obtained in this way contains a series of "spikes", so-called spikes , each spike representing a ligand injection with the following temperature change and re-adjustment of the temperature by the feedback device. The area under each spike corresponds to the amount of heat that is released or absorbed during this injection and can therefore be determined by integration as a function of time. The pattern of these binding heat effects can be analyzed as a function of the ligand / macromolecule molar ratio , whereby the thermodynamic parameters of the interaction studied can be determined. The samples should be degassed under vacuum before starting the experiment, as air bubbles within the cells can interfere with the measurements and lead to abnormal spectra.
See also
literature
- Friedrich Lottspeich, Joachim W. Engels (Ed.): Bioanalytik. 2nd Edition. Spectrum Academic Publishing House, 2006, ISBN 3-8274-1520-9 .
- R. O'Brien, JE Ladbury, BZ Chowdry: Isothermal titration calorimetry of biomolecules. In: SE Harding, BZ Chowdry (Ed.): Protein-Ligand interactions: hydrodynamics and calorimetry. Oxford University Press, 2000, ISBN 0-19-963746-6 .
- VP-ITC MicroCalorimeter User's Manual . 2001 ( PDF; 5.8 MB ( memento from June 22, 2015 in the Internet Archive )).
- T. Desphande: Isothermal Titration Calorimetry (ITC): Application in Drug Discovery.