Catalytically perfect enzymes
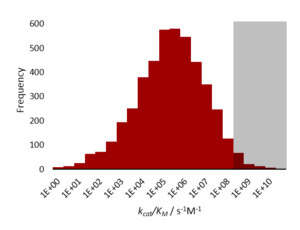
A catalytically perfect enzyme or kinetically perfect enzyme is an enzyme that catalyzes so efficiently that a catalytic reaction takes place whenever the enzyme and the corresponding substrate meet. The k cat / K m factor of such an enzyme is on the order of 10 8 to 10 9 M −1 s −1 . Thus, the reaction between substrate and enzyme is only limited by the rate of diffusion.
Examples of catalytically perfect enzymes are, for example, triose phosphate isomerase , carbonic anhydrase , acetylcholinesterase , catalase , fumarase , β-lactamase and superoxide dismutase .
history
A theory describing a diffusion-controlled reaction was taken up by Robert A. Alberty , Gordon Hammes and Manfred Eigen to find the maximum value for the catalytic efficiency of an enzyme-substrate reaction. This value is described by the k cat / K m factor. According to their investigations, the maximum value was 10 9 M −1 s −1 .
In 1972 it was observed that the dehydration of carbonic acid is catalyzed by α-carbonic anhydrase, the k cat / K m factor being 1.5 · 10 10 M −1 s −1 , which is the experimentally determined value by Alberty , Hammes and Eigen by far.
Prof. Kuo-Chen Chou and his colleagues perfected the previous model by Alberty, Hammes and Eigen by taking into account the spatial factor and the force field factor between the enzyme and its substrate. The more precise maximum value was now 10 10 M −1 s −1 . This model can explain the surprisingly high reaction rate.
mechanism
However, some enzymes show kinetics that run faster than the diffusion rate, which at first glance seems impossible. Various mechanisms have been proposed to explain this phenomenon:
Some enzymes are believed to accelerate catalysis by “pulling” their substrates towards them and aligning them with electrical fields. Based on Circe , a sorceress from Greek mythology who, according to legend , lured Odysseus ' men into her house and turned them into pigs, William P. Jencks coined the term Circe effect for this . Another theory suggests a quantum mechanical approach, whereby a proton or an electron can cross a potential barrier with the help of the tunnel effect , but this approach is considered contradictory, at least for protons. However, a tunneling effect for protons was observed with tryptamine . This fact suggests that the process of enzyme catalysis could be better described if one assumes the passage through a potential barrier; in the traditional model a substrate has to exceed a certain activation energy in order to leave its potential well.
Alberty Hammes Eigen model (AHE model)
The following equation based on the Debye-Smoluchowski theory is used to calculate the rate constant in the AHE model :
| Rate constant in the AHE model | |
| Diffusion coefficient , | |
| Avogadro's constant | |
| Reaction radius (sum of the radius of the "active hemisphere" and a substrate molecule, where r 0 = 5 Å) | |
| Interaction potential between a substrate molecule and the active site of an enzyme | |
| Boltzmann's constant | |
| Absolute temperature |
The active center is considered to be a hemisphere. It describes that all substrates that move in the opposite direction of the enzyme are aligned and attracted by an electric field in the direction of the active center located outside. The main protein, which is represented as the “active hemisphere”, represents a diffusion barrier that regulates the diffusion flow and the molecular forces. Thus, this model represents a special form of diffusion-controlled reaction, namely hemispherically symmetric diffusion. It has been proven that, according to the Alberty-Hammes Eigen model, the van der Waals forces are too low ( U 0 <3 kT ) because the active center is outside and the interactions between the active center and the substrate are decimated, which means that the main protein acts as a kind of "wall" and thus blocks the flow of substrate molecules to the active center and the enzymatic conversion is slowed down.
Chou model
To calculate the rate constant in the Chou model, the following equation was derived:
| Rate constant in the Chou model | |
| Diffusion coefficient , | |
| Avogadro's constant | |
| Hydrodynamic radius of the enzyme | |
| Enzyme unit | |
| Boltzmann's constant | |
| Absolute temperature | |
| Ratio of the concentration of substrate molecules on the entire surface of an enzyme molecule to the mass concentration |
In the Chou model, the force field and the geometric effect are taken into account. The speed factor in the Chou model is higher than in the Alberty-Hammes Eigen model, since the Van der Waals forces are greater. In the Chou model, the van der Waals forces are over 3 kT , since the active center is located within the enzyme and thus induces an increased interaction with the substrate, which means that the main protein acts as an "accelerator" and thus the flow of substrate molecules to the active center is accelerated. All substrates that are close to the enzyme are aligned and attracted by the electric field in the direction of the active center located within.
evolution
Due to natural selection , not many catalytically perfect enzymes exist. An increase in the catalytic reaction rate would only be advantageous if it does not exceed the diffusion rate. Thus, the rate of diffusion of the enzyme represents a physical limitation of evolution and can be represented as a global maximum in a fitness landscape . A fitness landscape represents the connection between the genotype and reproductive fitness , i.e. the number of offspring of an individual in his entire lifetime ( reproductive success ). The higher the landscape, the higher the replication rate and thus also the fitness. In living things, the rate of replication correlates with the rate of reproduction. Therefore, it is believed that perfect enzymes were created by random mutations and then spread, or the increased reaction rate was beneficial to an “ancestor” of the enzyme as part of another reaction mechanism.
Individual evidence
- ↑ a b Arren Bar-Even, Elad Noor: The Moderately Efficient Enzymes: Evolutionary and Physicochemical Trends Shaping enzymes parameter . In: Biochemistry . 50, No. 21, May 31, 2011, pp. 4402-4410. doi : 10.1021 / bi2002289 . PMID 21506553 .
- ^ Robert A. Alberty, Gordon G. Hammes: Application of the Theory of Diffusion-controlled Reactions to Enzyme Kinetics . In: The Journal of Physical Chemistry . 62, No. 2, February 1958, pp. 154-159. doi : 10.1021 / j150560a005 .
- ↑ Manfred Eigen, Gordon G. Hammes: Advances in Enzymology and Related Areas of Molecular Biology . Wiley-Blackwell, Hoboken (New Jersey) 1963, ISBN 978-0-470-12270-9 , pp. 1-38 .
- ↑ Kuo-Chen Chou, Shou-Ping Jiang: Studies on the rate of diffusion-controlled reactions of enzymes. Spatial factor and force field factor . In: Scientia Sinica . 27, No. 5, March 6, 1974, pp. 664-680. PMID 4219062 . ( Page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice.
- ↑ Kuo-Chen Chou: The kinetics of the combination reaction between enzymes and substrate Archived from the original on January 30, 2016. Information: The archive link has been inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. In: Scientia Sinica . 19, No. 4, 276, pp. 505-528. PMID 824728 . Retrieved January 30, 2016.
- ↑ Zi-Cai Li, Guo-Cheng Zhou: The quantitative relations between diffusion-controlled reaction rate and characteristic parameters in enzyme-substrate reaction systems. I. Neutral substrates Archived from the original on January 30, 2016. Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. In: Scientia Sinica . 19, No. 1, August 19753, pp. 117-136. PMID 1273571 . Retrieved January 30, 2016.
- ↑ Stryer: Biochemistry . 7th edition. Springer-Verlag, Berlin / Heidelberg 2013, ISBN 978-3-8274-2988-9 , pp. 238 .
- ↑ Mireia Garcia-Viloca, Jiali Gao, Martin Karplus and Donald G. Truhlar: In: Science . Jan. 9, 2004, Volume 303, No. 5655, pp. 186-195.
- ^ Mats MH Olsson, Per EM Siegbahn and A. Warshel: In: Journal of the American Chemical Society . March 10, 2004. Volume 126, No. 9, pp. 2820-2828.
- ↑ L. Masgrau, A. Roujeinikova, LO Johannissen, P. Hothi, J. Basran, KE Ranaghan, AJ Mulholland, MJ Sutcliffe, NS Scrutton and D. Leys: Atomic Description of an Enzyme Reaction Dominated by Proton Tunneling . In: Science . 312, No. 5771, 2006, pp. 237-241. PMID 16614214 .
- ^ A b Gua-Qiang Zhou, Wei-Zhu Zhong: Diffusion-controlled reactions of enzymes. A comparison between Chou's model and Alberty-Hammes-Eigen's model . In: Eur J Biochem . 128, No. 2-3, November 15, 1982, pp. 383-387. doi : 10.1111 / j.1432-1033.1982.tb06976.x . PMID 7151785 .
- ↑ Kostyčev Sergej Pavlovič: Textbook of plant physiology: First volume: Chemical physiology . Springer-Verlag, 2013, p. 567 ( full text in Google Book Search).

![{\ displaystyle \ mathrm {{\ mathit {k}} _ {AHE} = {\ frac {2 \ pi {\ mathit {D}} {\ mathit {N}} _ {A}} {1000 \ int \ limits _ {{\ mathit {r}} _ {0}} ^ {\ infty} \ exp \, [{\ mathit {U}} \! ({\ mathit {l)}} / {\ mathit {kT}} ] \, {\ frac {d {\ mathit {l}}} {{\ mathit {l}} ^ {2}}}}} \, (M ^ {- 1} \, s ^ {- 1}) }}](https://wikimedia.org/api/rest_v1/media/math/render/svg/9a24463700ad29e0b91a999779a4c540b17abb94)








![{\ displaystyle \ mathrm {{\ mathit {k}} _ {lim} = {\ frac {4 \ pi {\ mathit {D}} {\ mathit {N}} _ {A}} {1000 \ int \ limits _ {{\ mathit {R}} _ {0}} ^ {x} e ^ {\ mathit {U / kT}} \, {\ frac {d {\ mathit {r}}} {{\ mathit {r }} ^ {2}}}}} [1 - {\ tilde {g}} e ^ {{\ mathit {U}} ({\ mathit {R}} _ {0}) / {\ mathit {kT} }}] \, (M ^ {- 1} \, s ^ {- 1})}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/f1ea295db6f9b824aacd35adfff0193bca8f3515)



