Cationic demand
The cationic demand is a measure used to characterize suspensions and solutions . It is determined by a polyelectrolyte titration , the v. a. is used in the paper industry for the routine examination of pulp or pulp suspension .
The cationic requirement of a suspension is the amount of charge equivalents that are required to neutralize the charges of the dispersed / dissolved particles present in it. This is determined in a titration with a highly cationic or highly anionic polymer of short chain length as the titrand , of which the charge equivalent concentration is known.
As such titrant be in anionic templates usually a poly DADMAC , in cationic one Kaliumpolyvinylsulfat used.
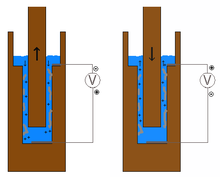
The end point of the titration is determined with a so-called Particle Charge Detector (PCD). The fine particles in the suspension adsorb on the Teflon surface of the PCD measuring cylinder. Raising and lowering the piston constantly creates a liquid suction that causes a streaming potential that is tapped as alternating voltage through contacts in the measuring cylinder. The end point of the titration is reached when the voltage U = 0. The result is given in microequivalents per milliliter of suspension (µeq / ml).