Kaufmann-Bucherer-Neumann experiments

The Kaufmann-Bucherer-Neumann experiments (1901–1915) examined the dependence of the inertial mass (or momentum ) of electrons on their speed . In the early days of the development of the special theory of relativity , these experiments were of great importance for the recognition of this then new theory. The results of these experiments were controversial for a long time and could only be fully verified decades after they were first carried out in terms of the special theory of relativity (cf. tests of the relativistic energy-momentum relationship and general tests of the special theory of relativity ).
prehistory
Henri Becquerel discovered the radioactive decay of a large number of chemical elements in 1896 . Then the resulting beta radiation was discovered, which was interpreted as the emission of negatively charged particles . These particles were later identified with the electron detected by Joseph John Thomson's experiments on cathode rays in 1897 .
Linked to this was the theoretical derivation of the so-called electromagnetic mass by JJ Thomson (1881). According to this, electromagnetic energy appears to increase the mass of a body. Thomson (1893) and George Frederick Charles Searle (1897) also calculated that this electromagnetic mass depends on the speed and becomes infinitely large when an electric charge moves with the speed of light relative to the ether . Also Hendrik Lorentz (1899, 1900) could derive such velocity dependence as a consequence of his theory of electrons. At this point the electromagnetic mass was called "apparent mass" and the unchanging Newtonian mass was called "true mass".
The (transversal) electromagnetic mass corresponded to the later developed concept of the " relativistic mass ", whereby the latter is valid for all other forms of energy in addition to the electromagnetic energy. These mass concepts are hardly used any more. Instead, the relativistic energy or momentum is used, which also includes the inaccessibility of the speed of light (symbol c) for massive particles, because the following applies:
That is why the Kaufmann-Bucherer-Neumann experiments can also be seen as early tests of the relativistic energy-momentum relationship . (For historical reasons, the terms “transversal” or “relativistic mass” will continue to be used below.)
Kaufmann's experiments
First experiments
With the cathode rays customary at the time , a maximum of 0.3c could have been achieved, which is why Walter Kaufmann used beta radiation (formerly known as "Becquerel rays ") with speeds of over 0.9c for his experiments. The decay of radium in an evacuated tube served as an electron source (Fig. 1). The charge to mass ratio of the particles was measured using electric and magnetic fields . These fields were aligned parallel to one another, so that the deflections they caused were perpendicular to one another. The impact of the particles on the photographic plate produced a deflection curve that matched a certain speed and mass. By reversing the electric field, two symmetrical curves were created, the center line of which determined the direction of the magnetic deflection. Since the electron charge was independent of changes in velocity, any change in the charge-to-mass ratio e / m must be the result of a change in mass or momentum.
Kaufmann published his first results in 1901 and was actually able to determine a decrease in the charge-to-mass ratio. This can only be explained if the mass or the momentum increases accordingly with greater speed. Based on the formula established by Searle (1897) for the dependence of electromagnetic energy on the speed of charged bodies, Kaufmann defined the increase in electromagnetic mass as a function of speed:
- .
Kaufmann noticed that this formula could not explain the results, so he assumed that the total mass of electrons mainly belongs to the "true mechanical mass", but only a smaller part belongs to the "apparent electromagnetic mass". However, he made two mistakes in the evaluation: On the one hand, Max Abraham was able to show that the above formula is only correct in the longitudinal direction, but the transverse direction is decisive for these experiments. Assuming the electron is a rigid sphere, the following applies here:
On the other hand, Kaufmann made a miscalculation when calculating the deflection curves. In a re-analysis in 1902, he corrected these errors and found agreement with Abraham's formula.
In 1902 and 1903 Kaufmann carried out further experiments under improved conditions. The results were taken by him as further confirmation of Abraham's theory and as evidence of the fully electromagnetic origin of the mass.
The increase in electron mass with speed was also confirmed by Hermann Starke , who in 1903 carried out experiments with cathode rays of about 0.3c.
Competing theories

In 1902 Max Abraham published a theory in which the electron was a rigid sphere, the charge of which is evenly distributed on its surface. As described above, he introduced the “transverse electromagnetic mass” in addition to the “longitudinal electromagnetic mass”. According to this theory, the entire electron mass would be of electromagnetic origin, while a mechanical mass no longer exists at all.
Lorentz (1899, 1904) also expanded his electron theory by introducing the Lorentz transformation , which showed that the electrons are subject to a shortening, the so-called length contraction, in the direction of movement. This gave rise to terms for electromagnetic mass that differed from Abraham's. Nevertheless, Lorentz was able to show that they agreed with Kaufmann's results as well as those of Abraham. In 1905, Henri Poincaré was able to develop Lorentz's theory further, so that from now on it was based on the principle of relativity , i.e. that is, fully coincided with the impossibility of determining an absolute, inertial motion.
Another theory was developed by Alfred Bucherer and Paul Langevin in 1904 . It differs from Lorentz's in that, together with the contraction in the direction of movement, an expansion occurs perpendicular to it, whereby the volume remains constant.
Finally, in 1905 Albert Einstein developed the special theory of relativity , which is still valid today , which included a change in mass due to the Lorentz transformation between inertial systems moving relative to one another. Despite completely different assumptions, the predictions of this theory correspond to those of Lorentz.
With regard to the transversal increase in mass, the following predictions of the respective theory resulted:
Experiments from 1905
In order to make a decision between these theories, Kaufmann conducted his experiments again with greater precision. From the comparison of the above formulas with his results, Kaufmann concluded that he had clearly refuted the Lorentz-Einstein formula, and thus the principle of relativity. Therefore, the only remaining theories are those of Abraham and Bucherer, which agreed almost equally well with the results. Lorentz was at a loss and wrote in a letter: "I am at the end of my Latin".
Shortly after Kaufmann had published his results and conclusions, however, they were subjected to a new analysis by Max Planck . In two papers published in 1906 and 1907, he could not find any experimental or calculation errors, but he showed that Kaufmann's results were not completely conclusive. Further extrapolation of the curves would result in the possibility of faster than light speeds. Thus, these measurements would not represent a final decision. And while Einstein admitted in 1907 that Kaufmann's results fit the theories of Abraham and Bucherer better than his own, the foundations of these theories are implausible and broad enough that they have a low probability of being correct.
More experiments
Bucherer
Adolf Bestelmeyer (1906–1907) criticized some technical aspects of Kaufmann's experiments, especially the use of parallel electric and magnetic fields. Therefore, he carried out experiments with cathode rays of about 0.3c himself. In doing so, he developed a velocity filter using perpendicular electric and magnetic fields. Similar methods had previously been used by JJ Thomson and Wilhelm Wien . In doing so, he received results for the charge-to-mass ratio that differed considerably from those of Kaufmann. Bestelmeyer added, however, that his data did not allow a decision between the theories.
For this reason Alfred Bucherer carried out new experiments in 1908 with beta rays of up to 0.7c using a velocity filter similar to that of Bestelmeyer (Fig. 4 & 5.) A radium source was located in the middle of a circular, charged capacitor, which was in turn was in a magnetic field. Only in the case of those rays that propagated in the direction α and had a certain speed did magnetic and electric fields compensate each other exactly so that they propagated in a straight line. After the rays left the capacitor, they were deflected by the magnetic field and hit the photographic plate.
For the final analysis, Bucherer used the formulas of Abraham and Lorentz-Einstein to calculate the charge-to-mass ratio for electrons at rest from the measured values . Since this ratio is constant in the resting state, the values must all lie on one line (FIG. 6). This was almost only the case with Lorentz-Einstein, while Abraham's data differed significantly. Therefore Bucherer concluded that the results had confirmed the relativity principle and thus the relativity theory or the "Lorentz-Einstein theory". Bucherer's results were received with relief and satisfaction by Lorentz, Einstein, and Hermann Minkowski .
Kurt Wolz , a student of Bucherer, carried out more extensive experiments in which he also found agreement with the Lorentz-Einstein formula (FIG. 7).
So although the majority of physicists accepted Bucherer's results, doubts remained. So there were objections, mainly from Bestelmeyer, with regard to the technical implementation, which led to a polemical dispute between Bucherer and Bestelmeyer in several publications. Bestelmeyer, for example, objected that a single experiment did not justify such far-reaching conclusions that exact data protocols were missing and that the influence of the uncompensated rays could not be ruled out. Wolz's experiments would not have invalidated his objections either.
Hupka
In contrast to Kaufmann and Bucherer, Karl Erich Hupka (1909) used cathode rays of up to 0.5c for his experiments. The rays generated by a copper cathode were accelerated by a field between the anode and cathode of an evacuated discharge tube. The anode served as a diaphragm through which the beams passed at constant speed and which cast the shadow image of two Wollaston wires onto a phosphorescent screen behind a second diaphragm. When a current was created behind the diaphragm, the rays were deflected and the silhouette shifted. The data agreed with the Lorentz-Einstein formula, but Hupka added that his experiment could not lead to a definitive decision. W. Heil then published some papers in which he analyzed and criticized the results. Hupka commented on this and defended his results and methods.
Neumann and Guye / Lavanchy
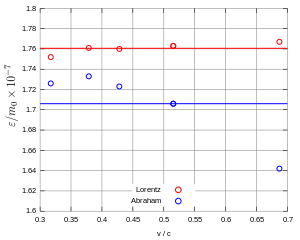
In 1914 Günther Neumann carried out new measurements using Bucherer's apparatus, which he further improved. He particularly responded to Bestelmeyer's objections, such as the question of non-compensated beams and extended data protocols. His experiment showed that here too the data on the charge-to-mass ratio according to the Lorentz-Einstein formula were approximately on a horizontal line, as required, while Abraham's data showed a clear curve (FIG. 8). Neumann concluded that his experiments agreed with those of Bucherer and Hupka, had definitively confirmed the Lorentz-Einstein formula in the range 0.4-0.7c, and had definitively refuted Abraham's formula. Since instrumental errors occurred in the range 0.7–0.8c, the deviation from the Lorentz-Einstein formula found in this range is not significant.
In 1915, Charles-Eugène Guye and Charles Lavanchy observed the deflection of cathode rays at speeds of 0.25-0.5c. The rays were accelerated in a tube with a cathode and an anode. The diaphragm on the anode created the beam, which was then deflected. The rays hit a screen on which the hits were photographed with a camera. Then they calculated the ratio of the transversal electromagnetic mass m T and “rest mass” m 0 and found agreement with the Lorentz-Einstein formula (Fig. 9). This confirmed Neumann's result even at lower speeds.
These experiments were taken as conclusive confirmations of the Lorentz-Einstein formula, so that Lorentz could write in 1915:
- Later experiments […] have confirmed the formula […] for the transverse electromagnetic mass, so that in all probability, the only objection that could be made against the hypothesis of the deformable electron and the principle of relativity has now been eliminated.
Further developments
Decades later, Zahn and Spees (1938) and Faragó and L. Jánossy (1957) argued that many of the assumptions made by the experimenters of the time regarding the nature of the electrons or the properties of the experimental setup were very imprecise. Just like Kaufmann's measurements, the Bucherer-Neumann experiments would only have shown a qualitative increase in momentum or mass, which were by no means precise enough to allow a decision between the competing theories.
While these experiments were controversial for a long time, the studies of the fine structure of the hydrogen lines by Karl Glitscher provided a clear confirmation of the Lorentz-Einstein formula and a refutation of all competitive theories as early as 1917. In order to derive the fine structure, the exact values for relativistic energy and momentum are necessary, which is not possible with Abraham's formula.
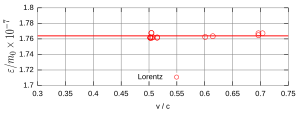
In order to bring about a final decision in electron deflection experiments as well, Rogers et al. (1940) carried out new experiments with improved equipment. The decay series of radium gives a spectrum of beta rays with a wide energy range. The earlier measurements by Kaufmann, Bucherer or Neumann used flat, parallel plate capacitors that did not allow the beta particles to be focused. Rogers et al. (Fig. 10), on the other hand, used an electrostatic spectrograph that enabled precise focusing. The spectrograph was constructed from two segments of two cylinders and was enclosed in an evacuated iron box. The beta rays were generated by a fine platinum wire coated with active radium. The rays hit a crack in front of a Geiger counter . The analysis showed that the measurement points on the curve for the ratio of transverse mass and rest mass were according to the Lorentz-Einstein formula (FIG. 11). An accuracy of 1% was achieved, which excluded Abraham's theory.
Modern tests
Today, the exact confirmation of the special theory of relativity in particle accelerators is already routine. Tests of the relativistic energy-momentum relationship
literature
- Michel Janssen, Matthew Mecklenburg: From classical to relativistic mechanics: Electromagnetic models of the electron . In: VF Hendricks, et al. (Ed.): Interactions: Mathematics, Physics and Philosophy . Springer, Dordrecht 2007, pp. 65-134.
- Hendrik Antoon Lorentz: The theory of electrons and its applications to the phenomena of light and radiant heat . BG Teubner, Leipzig / Berlin 1916.
- Arthur I. Miller: Albert Einstein's special theory of relativity. Emergence (1905) and early interpretation (1905-1911) . Addison-Wesley, Reading 1981, ISBN 0-201-04679-2 .
- Abraham Pais : Subtle is the Lord: The Science and the Life of Albert Einstein . Oxford University Press, 1982/2005, ISBN 0-19-280672-6 .
- Wolfgang Pauli : The Relativity Theory . In: Encyclopedia of Mathematical Sciences . 5, No. 2, 1921, pp. 539-776.
- Richard Staley: Einstein's generation . University Press, Chicago 2008, ISBN 0-226-77057-5 .
Web links
- Presentation on Kaufmann's experiment (PowerPoint; 1.3 MB)
- Schleif Roberts: What is the experimental basis of Special Relativity? 2006 (experimental tests of the SRT).
Individual evidence
Secondary source
- ↑ See Miller 1981, pp. 45-47.
- ↑ See Pais 2005, pp. 155–159
- ↑ See Miller 1981, pp. 47-54.
- ↑ See Staley 2008, pp. 223–233.
- ↑ a b See Miller 1981, pp. 55-67.
- ↑ See Staley 2008, pp. 229–233.
- ↑ a b See Janssen 2007, Section 4
- ↑ See Staley 2008, pp. 241–242
- ↑ See Miller 1981, pp. 228-232.
- ↑ See Staley 2008, pp. 242–244
- ↑ See Miller 1981, pp. 232-235
- ↑ See Staley 2008, pp. 244–250
- ↑ See Miller 1981, pp. 345-350.
- ↑ See Staley 2008, pp. 250-254.
- ↑ See Lorentz 1916, p. 339. " Later experiments [...] have confirmed the formula [...] for the transverse electromagnetic mass, so that, in all probability, the only objection that could be raised against the hypothesis of the deformable electron and." the principle of relativity has now been removed. "
- ↑ See Miller 1981, pp. 351-352
- ↑ See Janssen 2007, Section 7.
- ↑ See Pauli 1921, pp. 636–637
Primary sources
- ^ JJ Thomson: On the Effects produced by the Motion of Electrified Bodies . In: Philosophical Magazine . 11, No. 68, 1881, pp. 229-249.
- ^ GFC Searle: On the Steady Motion of an Electrified Ellipsoid . In: Philosophical Magazine . 44, No. 269, 1897, pp. 329-341.
- ↑ HA Lorentz: About the apparent mass of ions . In: Physikalische Zeitschrift . 2, No. 5, 1900, pp. 78-80.
- ↑ W. Kaufmann: The magnetic and electrical deflectability of the Bequerel rays and the apparent mass of the electrons . In: Göttinger Nachrichten . No. 2, 1901, pp. 143-168.
- ↑ W. Kaufmann: About the electromagnetic mass of the electron . In: Göttinger Nachrichten . No. 5, 1902, pp. 291-296.
- ↑ W. Kaufmann: The electromagnetic mass of the electron . In: Physikalische Zeitschrift . 4, No. 1b, 1902, pp. 54-56.
- ↑ W. Kaufmann: About the "electromagnetic mass" of electrons . In: Göttinger Nachrichten . No. 3, 1903, pp. 90-103.
- ↑ H. Starke: About the electrical and magnetic deflection of fast cathode rays . In: Negotiations of the German Physical Society . No. 13, 1903, pp. 241-250.
- ↑ M. Abraham: Dynamics of the Electron . In: Göttinger Nachrichten . 1902, pp. 20-41.
- ↑ M. Abraham: Principles of the dynamics of the electron . In: Physikalische Zeitschrift . 4, No. 1b, 1902, pp. 57-62.
- ↑ M. Abraham: Principles of the dynamics of the electron . In: Annals of Physics . 10, 1903, pp. 105-179.
- ↑ Hendrik Antoon Lorentz: Electromagnetic phenomena in a system that moves with any speed that cannot be reached by light . In: Otto Blumenthal, Arnold Sommerfeld (ed.): The principle of relativity. A collection of treatises (1913) 1904, pp. 6-26.
- ^ Henri Poincaré: Sur la dynamique de l'électron . In: Rendiconti del Circolo matematico di Palermo . 21, pp. 129-176. See also German translation .
- ↑ AH Bucherer: Mathematical introduction to electron theory. Teubner, Leipzig 1904, p. 57.
- ↑ Albert Einstein: On the electrodynamics of moving bodies . In: Annals of Physics . 322, No. 10, 1905, pp. 891-921. doi : 10.1002 / andp.19053221004 .
- ↑ Walter Kaufmann: About the constitution of the electron . In: Session reports of the Royal Prussian Academy of Sciences . 45, 1905, pp. 949-956.
- ↑ Walter Kaufmann: About the constitution of the electron . In: Annals of Physics . 324, No. 3, 1906, pp. 487-553.
- ↑ Max Planck: The Kaufmann measurements of the deflectability of β-rays and their significance for the dynamics of electrons . In: Physikalische Zeitschrift . 7, 1906, pp. 753-761.
- ↑ M. Planck: Addendum to the discussion of Kaufmann's deflection measurements . In: Negotiations of the German Physical Society . tape 9 , no. 14 , 1907, pp. 301-305 .
- ↑ Albert Einstein: About the principle of relativity and the conclusions drawn from it . In: Yearbook of radioactivity and electronics . 4, 1908, pp. 411-462.
- ↑ A. Bestelmeyer: Specific charge and speed of the cathode rays generated by X-rays . In: Annals of Physics . 327, No. 3, 1907, pp. 429-447. doi : 10.1002 / andp.19073270303 .
- ↑ AH Bucherer: Measurements on Becquerel rays. The experimental confirmation of the Lorentz-Einstein theory . In: Physikalische Zeitschrift . 9, No. 22, 1908, pp. 755-762.
- ↑ AH Bucherer: The experimental confirmation of the principle of relativity . In: Annals of Physics . 333, No. 3, 1909, pp. 513-536. doi : 10.1002 / andp.19093330305 .
- ↑ Kurt Wolz: The determination of e / m0 . In: Annals of Physics . 335, No. 12, 1909, pp. 273-288. doi : 10.1002 / andp.19093351206 .
- ↑ AH Bestelmeyer: Comments on the treatise by Mr. AH Bucherer: The experimental confirmation of the principle of relativity . In: Annals of Physics . 335, No. 11, 1909, pp. 166-174. doi : 10.1002 / andp.19093351105 .
- ↑ AH Bucherer: Answer to the criticism of Mr. E. Bestelmeyer regarding my experimental confirmation of the principle of relativity . In: Annals of Physics . 335, No. 11, 1909, pp. 974-986. doi : 10.1002 / andp.19093351506 .
- ↑ AH Bestelmeyer: Reply to the answer of Mr. AH Bucherer . In: Annals of Physics . 337, No. 6, 1910, pp. 231-235. doi : 10.1002 / andp.19103370609 .
- ↑ AH Bucherer: Reply to the remarks of Mr. A. Bestelmeyer . In: Annals of Physics . 338, No. 14, 1910, pp. 853-856. doi : 10.1002 / andp.19103381414 .
- ↑ E. Hupka: Contribution to the knowledge of the inertial mass of moving electrons . In: Annals of Physics . 336, No. 1, 1910, pp. 169-204. bibcode : 1909AnP ... 336..169H . doi : 10.1002 / andp.19093360109 .
- ↑ W. Heil: Discussion of the experiments on the inert mass of moving electrons . In: Annals of Physics . 336, No. 3, 1910, pp. 519-546. bibcode : 1910AnP ... 336..519H . doi : 10.1002 / andp.19103360305 .
- ↑ E. Hupka : On the question of the inert mass of moving electrons . In: Annals of Physics . 338, No. 12, 1910, pp. 400-402. bibcode : 1910AnP ... 336..519H . doi : 10.1002 / andp.19103360305 .
- ↑ W. Heil: For the discussion of Hupka's experiments on the inert mass of moving electrons . In: Annals of Physics . 338, No. 12, 1910, pp. 403-413. bibcode : 1910AnP ... 338..403H . doi : 10.1002 / andp.19103381210 .
- ↑ Günther Neumann: The inert mass of fast moving electrons . In: Annals of Physics . 350, No. 20, 1914, pp. 529-579. bibcode : 1914AnP ... 350..529N . doi : 10.1002 / andp.19143502005 .
- ^ CE Guye, C. Lavanchy: Vérification expérimentale de la formule de Lorentz-Einstein par les rayons cathodiques de grande vitesse . In: Compt. Rend. Acad. Sci. . 161, 1915, pp. 52-55.
- ^ CE Guye, C. Lavanchy: Vérification expérimentale de la formule de Lorentz-Einstein par les rayons cathodiques de grande vitesse . In: Archives des sciences physiques et naturelles . 42, 1915, p. 286ff.
- ^ CT Zahn, AA Spees: A Critical Analysis of the Classical Experiments on the Variation of Electron Mass . In: Physical Review . 53, 1938, pp. 511-521. bibcode : 1938PhRv ... 53..511Z . doi : 10.1103 / PhysRev.53.511 .
- ^ PS Faragó, L. Jánossy: Review of the experimental evidence for the law of variation of the electron mass with velocity . In: Il Nuovo Cimento . 5, No. 6, 1957, pp. 379-383. doi : 10.1007 / BF02856033 .
- ↑ Karl Glitscher: Spectroscopic comparison between the theories of the rigid and the deformable electron . In: Annals of Physics . 357, No. 6, 1917, pp. 608-630. doi : 10.1002 / andp.19173570603 .
- ^ MM Rogers et al .: A Determination of the Masses and Velocities of Three Radium B Beta-Particles . In: Physical Review . 57, 1940, pp. 379-383. doi : 10.1103 / PhysRev.57.379 .




![\ phi (\ beta) = {\ frac {3} {4 \ beta ^ {{2}}}} \ left [{\ frac {1} {\ beta}} \ lg {\ frac {1- \ beta} {1+ \ beta}} + {\ frac {2} {1- \ beta ^ {{2}}}} \ right], \; \ beta = {\ frac {v} {c}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a76587532865bf01653d4c4f8b77572cb8006ea9)