Kröhnke pyridine synthesis
The Kröhnke pyridine synthesis is a name reaction in organic chemistry that was named after its discoverer, the German chemist Fritz Kröhnke (1903–1981). It is used for the synthesis of pyridine derivatives starting from unsubstituted pyridine. The reaction takes place via several intermediate stages, with α, β-unsaturated ketones , among others , being used.
Overview reaction
The Kröhnke pyridine synthesis provides pyridine derivatives with the substitution of three hydrogen atoms in the starting compound pyridine. The substituents R 1 , R 2 and R 3 are organic radicals .
For example, these organic radicals can be methyl groups , as the following overview shows. However, the residues bound in the target molecule do not necessarily have to be the same.
Reaction mechanism
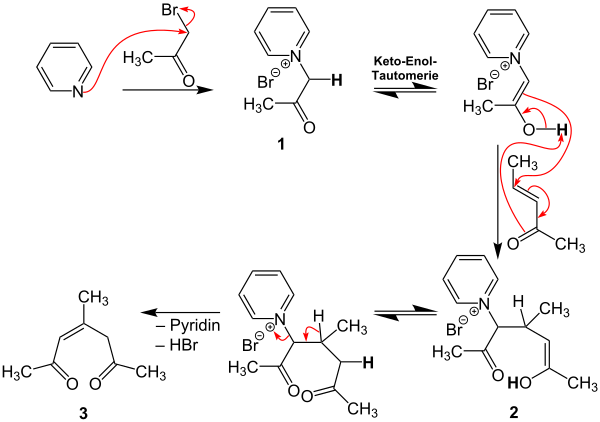
A possible reaction mechanism for the Kröhnke pyridine synthesis according to the second overview reaction is described below. In this example, R 1 , R 2 and R 3 are methyl radicals and thus the reaction product 2,4,6-collidine .
First, pyridine reacts with bromoacetone to form intermediate 1 . A keto-enol tautomerism is followed by the addition of ( E ) -3-penten-2-one and intermediate stage 2 is formed . After another keto-enol tautomerism, pyridine and HBr are split off, so that intermediate 3 is formed. The reaction takes place in the presence of a mixture of ammonium acetate and acetic acid , which are in the following equilibrium :
This is how ammonia is supplied for the next reaction step:
First, ammonia is added to intermediate level 3 and intermediate level 4 is created via tautomerism . After the water has split off , the ring closes. Finally, water is split off again with aromatization and 2,4,6-trimethylpyridine ( 5 ) is formed.
Individual evidence
- ↑ Wilfried Zecher, Fritz Kröhnke: A new synthesis of substituted pyridines, I. Principles of synthesis . In: Chemical Reports . tape 94 , no. 3 , 1961, pp. 690-697 , doi : 10.1002 / cber.19610940317 .
- ↑ Jie Jack Li: Name reactions: A collection of detailed mechanisms and synthetic applications . 5th ed.Springer, Cham 2014, ISBN 978-3-319-03979-4 , pp. 413-414 , doi : 10.1007 / 978-3-319-03979-4 .
- ↑ Bradford P. Mundy; Michael G. Ellerd; Frank G. Favaloro: Name reactions and reagents in organic synthesis . 2nd ed. Wiley, Hoboken (NJ) 2005, ISBN 0-471-73987-1 , pp. 304 .
- ↑ Zerong Wang: Comprehensive organic name reactions and reagents Volume 2 . John Wiley, Hoboken (NJ) 2009, ISBN 978-0-470-28663-0 , pp. 1695-1698 .