Cresol red
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Cresol red | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 21 H 18 O 5 S | |||||||||||||||
| Brief description |
green to dark red crystalline powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 382.43 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
290 ° C (decomposition) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Cresol red is a triphenylmethane dye and belongs to the group of sulfonphthaleins. It is used as a pH indicator . Its phthalein analogue is o -cresolphthalein . The bromocresol purple can be represented by bromination .
properties
There are two color change points:
- pH 0.2-1.8: color change from red to yellow
- pH 7.0-8.8: color change from yellow to violet
Cresol red contains two hydroxyl groups and a less stable sultone ring . This ring is split in an aqueous medium, and after a rearrangement the quinoid yellow colored form of the dye is formed. In a strongly acidic environment (pH <1.8) the quinoid system is protonated, which causes the solution to turn red. In a basic medium (pH = 7.0–8.8) the hydroxyl group is deprotonated and the solution turns purple.
| species | H 2 In | In - | In 2− |
|---|---|---|---|
| structure |  |
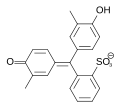 |

|
| pH | <1.8 | 2.0-7.0 | > 8.8 |
| colour | red | yellow | violet |
use
Cresol red is used as an indicator in acid-base titrations. In most cases, only the second transition range (pH = 7.0–8.8) is used for the indication.
Individual evidence
- ↑ a b c data sheet Cresol red from Sigma-Aldrich , accessed on April 7, 2011 ( PDF ).
- ↑ a b Data sheet cresol red (PDF) from Merck , accessed on February 20, 2010.
- ↑ Entry on cresol purple. In: Römpp Online . Georg Thieme Verlag, accessed on September 29, 2014.
- ^ I. Kolthoff, C. Rosenblum: Acid-Base Indicators , MacMillan, New York 1937, pp. 108-109.
- ↑ RW Sabnis: Handbook of Acid-Base Indicators , CRC Press, 2008, p. 105.
- ^ Udo R. Kunze: Basics of quantitative analysis , 3rd edition, Georg Thieme Verlag, Stuttgart 1990, p. 96.
