Copper-indium-gallium-diselenide
| Crystal structure | |||||||
|---|---|---|---|---|---|---|---|
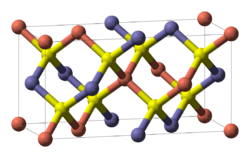
|
|||||||
| __ copper __ selenium __ indium or gallium | |||||||
| Space group |
I 4 2 d (No. 122) |
||||||
| General | |||||||
| Surname | Copper-indium-gallium-diselenide | ||||||
| other names |
CIGS |
||||||
| Ratio formula | CuIn x Ga (1 − x) Se 2 | ||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | variable | ||||||
| Physical state |
firmly |
||||||
| density |
≈ 5.7 g / cm 3 |
||||||
| Melting point |
990-1070 ° C ( x = 1-0) |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Copper-Indium-Gallium-Diselenide (short name CIGS , chemical formula CuIn x Ga (1 − x) Se 2 ) is an I-III-VI compound semiconductor consisting of copper (Cu), indium (In), gallium (Ga) and Selenium (Se). It is a mixed crystal and consists of the two starting substances copper-indium-diselenide , often also referred to as CIS , and copper-gallium-diselenide , often also referred to as CGS . The chemical bond is due to their crystal structure the chalcopyrites with the space group I 4 2 d (space group no. 122) assigned. Various material properties can be influenced via the mixing ratio of the two starting substances, expressed by the factor x in the chemical name, which can be in the range 0 to 1. Depending on the mixing ratio , the band gap is 1.02 eV for x = 1 (pure copper-indium-diselenide) to 1.7 eV for x = 0 (pure copper-gallium-diselenide).
CIGS is used, among other things, as a material in thin-film CIGS solar cells . The use of this semiconductor material in photovoltaics can be optimized by selecting the band gap accordingly.
Individual evidence
- ↑ LTS Chem: MSDS ( Memento of the original from December 3, 2013 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ a b T. Tinoco, C. Rincón, M. Quintero, G. Sánchez Pérez: Phase Diagram and Optical Energy Gaps for CuInyGa1 − ySe2 Alloys . In: Physica Status Solidi (a) . 124, No. 2, 1991, pp. 427-434. doi : 10.1002 / pssa.2211240206 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ DOE Solar Energy Technologies Program Peer Review (PDF; 1.1 MB) US department of energy 2009. Accessed February 10, 2011.