L-selectride
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | L-selectride | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 12 H 28 BLi | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 190.10 g mol −1 | ||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
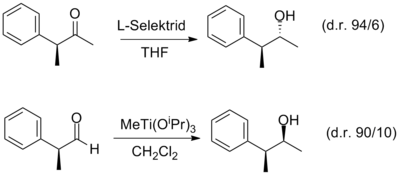
L-selectride is a sterically hindered and therefore selective reducing agent for ketones and the brand name of the Sigma-Aldrich company for lithium tri- sec- butyl (hydrido) borate. It is available as a solution in THF . The brand name is much more common than the systematic name. It is used, for example, in permutation reactions . A comparison between the reactions carried out by L-selectride and MeTi (O i Pr) 3 clarifies the background of a permutation reaction .
The reduction is usually carried out at low temperatures (−78 ° C). However, cases are known in which an increase in temperature leads to better stereoselectivity and even the reversal of selectivity.
Individual evidence
- ↑ a b Data sheet L-Selectride ® solution, 1.0 M in THF from Sigma-Aldrich , accessed on January 21, 2012 ( PDF ).
- ↑ Parts of the labeling of hazardous substances relate to the hazards caused by the solvent.
- ↑ Jerry March: Advanced Organic Chemistry , John Wiley & Sons, New York 1985, ISBN 0-471-88841-9 .
- ^ Kai Oesterreich, Dietrich Spitzner, Tetrahedron , 2002 , 58 ; 4331-4334, doi: 10.1016 / S0040-4020 (02) 00336-8 .