Mass excess
The mass excess ( English mass excess ) or mass excess of a nuclide is an auxiliary quantity formed from the atomic mass and the number of nucleons . It facilitates calculations in which the direct use of the atomic mass values would lead to inconveniently large numbers.
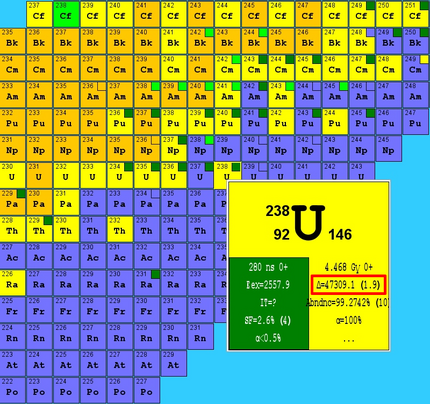
The mass excess is usually given as energy in the unit keV . (Large delta) is often used as a symbol .
Until 1993, the mass excesses were published in common tables instead of the atomic masses determined from measurement results. Although these tables have contained both data since 1995, many specialists in areas such as the calculation of nuclear reactors still prefer the excess of mass. The core data viewer JANIS 4 from NEA (as of 2016), for example, does not show the mass, but only the mass excess of each nuclide. The atomic weights used in chemistry are also calculated from the excess mass.
The quantities mass excess (or mass excess) and mass defect - or at least their names - are sometimes confused in scientific literature. While the mass defect has an obvious physical meaning as the equivalent of the binding energy of an atomic nucleus, the mass excess is to be regarded as a useful mathematical auxiliary variable.
definition
The mass excess is defined as the difference between the actual atomic mass and a fictitious mass formed with the number of nucleons of the nuclide and the atomic mass unit :
- .
Because of the mass-energy equivalence can this mass difference using the speed of light according to convert into its equivalent energy with the same letter will be referred to:
- .
This explains the usual specification of the excess mass in the energy unit keV .
In the literature, even in the references Atomic mass regular evaluations , are usually found only the short ( "lax") Definition: .
Range of values

The mass excess of all known nuclides is between approx. −90,000 keV ( 124 Te ) and approx. +200,000 keV ( 295 Og ). Due to the definition of the atomic mass unit, the mass excess of 12 C is exactly 0 keV. The mass excesses of all stable nuclides above 16 O are negative (see figure). This means that for the nuclide 16 O and all heavier stable nuclides the numerical value A u of the atomic mass is somewhat smaller than the nucleon number A, e.g. B. m ( 16 O) = 15.994915 u. Knowing this can be helpful in practice (visual inspection for gross data errors).
The atomic masses themselves have only been included in the atomic mass evaluation data lists since 1983 , in the unit µu.
Connection with the mass defect
The definition of the mass defect is
- .
The connection between mass defect and mass excess is therefore
- .
The number of neutrons , the number of protons and the sum of both mean the number of nucleons, the mass of a neutron and the atomic mass of the light hydrogen .
Calculation of the Q-value of a nuclear reaction from the excess mass
The mass excess is useful for calculating the energy balance of a nuclear reaction . With the excess mass of all the particles involved in the nuclear reaction, the energy balance, the Q value, can be calculated without differences between excessively large numbers occurring. Let and be the masses of the reacting particles and and the masses of the product particles , then the mass balance of the nuclear reaction
and the energy balance, the Q value
- .
With the excess mass of the four particles and taking into account the conservation of the total number of nucleons, that is
- ,
surrendered
- .
Individual evidence
- ^ A b Janis 4 - Java-based Nuclear Data Information System
- ↑ W. Ober: pregalactic nucleosynthesis in massive objects. Dissertation U. Göttingen 1981, page 62
- ↑ U. Quade: Measurement of the yields of the easy cleavage products from the reaction 233U (n-th, f) with an ionization chamber . Dissertation LMU Munich 1983, page 185, 187
- ↑ L. Schweikhard, D. Neidherr, K. Blaum: New radon isotope discovered. Physics in Our Time Volume 40, Issue 4 (2009), page 175
- ^ Josef Mattauch : Units of measurement for atomic weights and nuclide masses. In: Journal of Nature Research A . 13, 1958, pp. 572-596 ( online ). ; here page 573
- ↑ DC Giancoli: Physics: text and exercise book . 3rd edition, Pearson Studium 2010, ISBN 978-3-86894-023-7 , page 1436
- ↑ a b Harry Friedmann: Introduction to Nuclear Physics . Wiley-VCH, Weinheim, Bergstr 2014, ISBN 978-3-527-41248-8 , pp. 97 (XII, 481 pp., Limited preview in Google Book Search [accessed January 8, 2017]).
- ↑ Detlef Kamke , Klaus Krämer: Physical basics of the units of measurement: With an appendix on error calculation . 1st edition. Teubner, Stuttgart 1977, ISBN 3-519-03015-2 , pp. 80–82 (218 pages, limited preview in Google Book Search [accessed January 3, 2017]).
- ↑ a b Georges Audi, Aaldert Wapstra : The 1993 atomic mass evaluation: (I) Atomic mass table. In: Nuclear Physics A , Volume 565 (1993) pp. 1-65, doi: 10.1016 / 0375-9474 (93) 90024-R
- ↑ JR de Laeter et al. : Atomic weights of the elements: Review 2000 (IUPAC technical report) . In: Pure and applied chemistry . tape 75 , no. 6 , 2003, p. 683-800 ( online [PDF; accessed March 27, 2018]).
- ↑ a b A. H. Wapstra, G. Audi: The 1983 atomic mass evaluation: (I). Atomic mass table . In: Nuclear Physics A . tape 432 , no. 1 , 1985, pp. 1-54 , doi : 10.1016 / 0375-9474 (85) 90283-0 .



























