McFadyen-Stevens reaction
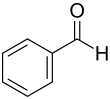
The McFadyen-Stevens reaction (also known as McFadyen-Stevens reduction , McFadyen-Stevens synthesis or McFadyen-Stevens aldehyde synthesis ) is a name reaction in organic chemistry . The reaction was named after its discoverers, the chemists John S. McFayden and Thomas S. Stevens. This synthesis produces aromatic or heteroaromatic aldehydes . A natural occurrence of aromatic aldehydes is bitter almond oil , the main component of which is benzaldehyde . Bitter almond oil is obtained from apricot kernels. Benzaldehyde is an aromatic aldehyde and can be synthesized using the McFadyen-Stevens reaction.
Overview reaction
A hydrazide is decomposed with sodium carbonate in the heat, whereby an aromatic (R 1 = aryl group ) or heteroaromatic aldehyde (R 1 = heteroaryl group ) is formed.
The R 2 is an aryl radical, e.g. B. a phenyl radical .
Reaction mechanism
One possible reaction mechanism is carried out with hydrazide 1 :
The hydrazide 1 is reacted with sodium carbonate , in which case the carbonate acts as a base and deprotonates a nitrogen atom. The deprotonated hydrazide 2 now forms the azo compound 3 by shifting the hydrogen, with splitting off of the sulfone group (an azo compound is e.g. diimine ). An aldehyde (R 1 = heterocyclic radical or aryl radical ) can now be formed from 3 with elimination of nitrogen .
Individual evidence
- ↑ Z. Wang (Ed.): Comprehensive Organic Name Reactions and Reagents, 2 Volume Set . John Wiley & Sons, Hoboken, NJ 2009, ISBN 978-0-470-28663-0 , p. 1852.
- ↑ John S. McFayden, Thomas S. Stevens: A new method for the conversion of acids into aldehydes . In: Journal of the Chemical Society . No. 128 , 1936, pp. 584-587 , doi : 10.1039 / JR9360000584 ( PDF ).
- ↑ a b Z. Wang (Ed.): Comprehensive Organic Name Reactions and Reagents, 2 Volume Set , John Wiley & Sons, Hoboken, NJ 2009, ISBN 978-0-470-28663-0 , p. 1853.
- ↑ BP Mundy, MG Ellerd and FG Favaloro Jr. (ed.): Name Reactions and Reagents in Organic Synthesis, Secound Edition . John Wiley & Sons, Hoboken, NJ 2005, ISBN 0-471-22854-0 , p. 412.