Methylenecitric acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
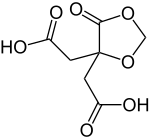
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Methylenecitric acid | ||||||||||||||||||
| Molecular formula | C 7 H 8 O 7 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 204.13 g mol −1 | ||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
The active pharmaceutical ingredient methylenecitric acid was first produced by Schering in 1901 by condensing formaldehyde with citric acid to form the dibasic methylencitric acid (a γ- lactone ). This process was described somewhat modified by Rudolph Berendes in the Bayer patent of October 9, 1902.
Citarin, Helmitol
The disodium salt was marketed under the Bayer brand name Citarin and the urotropin salt under the name Helmitol (also known as "Neu-Urotropin").
When stoichiometric amounts of concentrated alcoholic solutions of methylenecitric acid and hexamethylenetetramine are added together, the latter separates out quantitatively as a white powder with a melting point of 175-176 ° C.
Web links
- Arthur Eichengrün : Pharmazeutische Zeitung 47 , 857, 866–867 (1902) ( digitized version )
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Schering patent DE129255 of April 3, 1901 . - US patent US699422 dated October 29, 1901
- ↑ Bayer patent DE150949 of October 9, 1902 . - R. Berendes, U.S. Patent 722275, filed November 22, 1902 . - R. Berendes, U.S. Patent 715,239, filed September 4, 1902
- ↑ Word mark Citarin, Rg.Nr. 56353 of May 31, 1902 - deleted August 1, 2011
- ↑ R. Berendes: Citarin and Helmitol . In: Reports of the Deutsche Pharmaceutische Gesellschaft , 13 , 376 (1903).
- ↑ K. Winterfeld: Anhydromethylene citric acid hexamethylenetetramine - Helmitol . In: Practical course in organic preparative pharmaceutical chemistry . 4th edition. Theodor Steinkopff, Dresden and Leipzig 1955, p. 66-67 .