Meyer-Schuster rearrangement
The Meyer-Schuster rearrangement is a chemical reaction in which acid-catalyzed secondary and tertiary propargylic alcohols (R 1 = H or organyl group , R 2 = H or organyl group, R 3 = H or organyl group) rearrange to form α, β-unsaturated ketones . With a terminal alkyne group, α, β-unsaturated aldehydes are formed . The name reaction was discovered and published by Kurt H. Meyer and Kurt Schuster in 1922.
Several reviews on the Meyer-Schuster rearrangement have been published.
The base-catalyzed variant of the rearrangement is known as the Faworski reaction .
mechanism
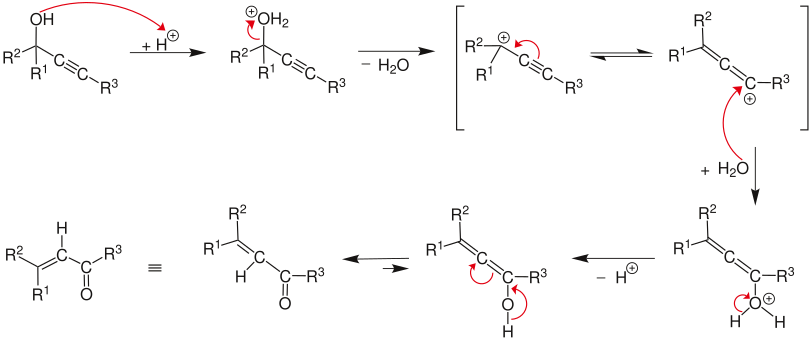
The reaction mechanism begins with the protonation of the alcohol, whereby water is eliminated in an E1 reaction and the allene is formed from the alkyne . By attack of the water molecule on the carbocation and subsequent deprotonation followed by tautomerism , an α, β-unsaturated carbonyl compound is formed.
The reaction mechanism has been described by Edens et al. examined. They found three characteristic steps: (1) the rapid protonation of oxygen, (2) the slow, rate-limiting step of the sigmatropic 1,3-rearrangement of the protonated hydroxyl group, and the keto-enol tautomerism, followed by rapid deprotonation.
In an investigation of the rate-limiting step of the Meyer-Schuster rearrangement, Andres et al. that the driving force of the reaction is the irreversible formation of the unsaturated carbonyl compound via the carbonium ion. They also showed that the reaction is aided by the solvent used. This was further elucidated by Tapi et al. who showed that the formation of solvent cages stabilizes the transition state.
Rupe rearrangement
The reaction of tertiary alcohols (R 4 , R 5 = organyl group ) which contain an alkyne group in the α-position does not lead to the expected alcohols but to α, β-unsaturated ketones via an enyne intermediate . For tertiary alcohols, this reaction takes place in competition with the Meyer-Schuster rearrangement and is referred to as the Rupe rearrangement (after Hans Rupe ). In the first step, the alcohol is protonated, with water being split off. A propargylation occurs, which is deprotonated and reacts to an enyne. After protonation of this intermediate stage and the reaction with water, the α, β-unsaturated ketone is formed with tautomerization.
Catalysts
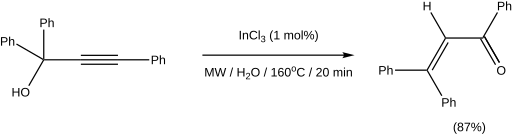
In the case of tertiary alcohols, the conditions of the traditional Meyer-Schuster rearrangement using strong acids as a catalyst lead to side reactions such as the Rupe rearrangement. By using transition metal and Lewis acids as catalysts, the reaction can be carried out under milder conditions, for example with ruthenium- and silver-containing catalysts. Carieno et al. reported the use of microwave irradiation with InCl 3 as a catalyst, which led to excellent yields and short reaction times , and remarkable stereoselectivity . An example from the publication is as follows:
Applications
The Meyer-Schuster rearrangement has a number of applications, from the conversion of ω-alkyne-ω-carbinol lactams into enamides with PTSA as a catalyst to the synthesis of α, β-unsaturated thioesters from γ-sulfur-substituted propargyl alcohols to the rearrangement of 3-alkyne-3-hydroxy-1 H - isoindoles under mildly acidic conditions to form α, β-unsaturated carbonyl compounds. One of the most interesting applications is the synthesis of a partial structure of paclitaxel in a diastereoselective synthesis that leads only to the E -alkene.
The synthesis step shown above takes place in 70% yield, and even in 91% yield if the by-product is converted into the Meyer-Schuster product in another step.
Individual evidence
- ↑ Kurt H. Meyer, Kurt Schuster: Rearrangement of tertiary ethynyl carbinols in unsaturated ketones. In: Reports of the German Chemical Society (A and B series). 55, 1922, pp. 819-823, doi : 10.1002 / cber.19220550403 .
- ^ A b S. Swaminathan, KV Narayanan: Rupe and Meyer-Schuster rearrangements. In: Chemical Reviews. 71, 1971, pp. 429-438, doi : 10.1021 / cr60273a001 .
- ↑ SA Vartanyan, Sh O Babanyan: rearrangement OF Acetylenic COMPOUNDS WITH PARTICIPATION OF THE π-electrons OF THE TRIPLE BOND. In: Russian Chemical Reviews. 36, 1967, pp. 670-686, doi : 10.1070 / RC1967v036n09ABEH001681 .
- ^ Douglas A. Engel, Gregory B. Dudley: The Meyer-Schuster rearrangement for the synthesis of α, β-unsaturated carbonyl compounds. In: Organic & Biomolecular Chemistry. 7, 2009, p. 4149, doi : 10.1039 / b912099h .
- ^ Li, JJ In Meyer-Schuster rearrangement ; Name Reactions: A Collection of Detailed Reaction Mechanisms; Springer: Berlin, 2006; pp 380-381. ( doi : 10.1007 / 978-3-642-01053-8_159 )
- ↑ László Kürti and Barbara Czakó: Strategic Applications of Named Reactions in Organic Synthesis: Background and Detailed Mechanisms , Elsevier Academic Press, 2005, pp. 284-285, ISBN 978-0-12-429785-2 .
- ↑ Edens, M .; Boerner, D .; Chase, CR; Nass, D .; Schiavelli, MD J. Org. Chem. 1977 , 42 , 3403-3408. ( doi : 10.1021 / jo00441a017 )
- ↑ Andres, J .; Cardenas, R .; Silla, E .; Tapia, O. J. Am. Chem. Soc. 1988 , 110 , 666-674. ( doi : 10.1021 / ja00211a002 )
- ↑ Tapia, O .; Lluch, JM; Cardena, R .; Andres, J. J. Am. Chem. Soc. 1989 , 111 , 829-835. ( doi : 10.1021 / ja00185a007 )
- ↑ Rupe, H .; Kambli, E. Helv. Chim. Acta 1926 , 9 , p. 672 ( doi : 10.1002 / hlca.19260090185 ).
- ^ Li, JJ In Rupe rearrangement ; Name Reactions: A Collection of Detailed Reaction Mechanisms; Springer: Berlin, 2006; Pp. 513-514 ( doi : 10.1007 / 978-3-642-01053-8_224 ).
- ↑ László Kürti and Barbara Czakó: Strategic Applications of Named Reactions in Organic Synthesis: Background and Detailed Mechanisms , Elsevier Academic Press, 2005, ISBN 978-0-12-429785-2 , pp. 284-285.
- ↑ Cadierno, V .; Crochet, P .; Gimeno, J. Synlett 2008 , 1105-1124. ( doi : 10.1055 / s-2008-1072593 )
- ↑ Sugawara, Y .; Yamada, W .; Yoshida, S .; Ikeno, T .; Yamada, T. J. Am. Chem. Soc. 2007 , 129 , 12902-12903. ( doi : 10.1021 / ja074350y )
- ↑ Cadierno, V .; Francos, J .; Gimeno, J. Tetrahedron Lett. 2009 , 50 , 4773-4776. ( Doi : 10.1016 / j.tetlet.2009.06.040 )
- ↑ Chihab-Eddine, A .; Daich, A .; Jilale, A .; Decroix, B. J. Heterocycl. Chem. 2000 , 37 , 1543-1548. ( Doi : 10.1002 / jhet.5570370622 )
- ↑ Yoshimatsu, M .; Naito, M .; Kawahigashi, M .; Shimizu, H .; Kataoka, T. J. Org. Chem. 1995 , 60 , 4798-4802. ( Doi : 10.1021 / jo00120a024 )
- ↑ Omar, EA; Tu, C .; Wigal, CT; Braun, LL J. Heterocycl. Chem. 1992 , 29 , 947-951. ( Doi : 10.1002 / jhet.5570290445 )
- ↑ Crich, D .; Natarajan, S .; Crich, JZ Tetrahedron 1997 , 53 , 7139-7158. ( Doi : 10.1016 / S0040-4020 (97) 00411-0 )