Miller-Dieker Syndrome
| Classification according to ICD-10 | |
|---|---|
| Q93.5 | Chromosomal abnormality |
| ICD-10 online (WHO version 2019) | |
When Miller-Dieker syndrome (MDS, also Miller Dieker- lissencephaly , or 17-p-syndrome ) is a disease as a result of a chromosomal abnormality in chromosome 17 . As a result, the cerebrum develops badly in the embryonic phase. Miller-Dieker syndrome is often associated with other developmental defects. It was first described in 1963 by James Quinter Miller and in 1969 by Hans Dieker.
Epidemiology
Miller-Dieker syndrome is a rare disease and occurs in fewer than 1.2: 100,000 live births. In 20% of the cases there is a balanced translocation of the affected gene segment in the parents. 80% of the cases represent a new mutation . If the parents have a balanced translocation, the risk of a Miller-Dieker syndrome in the child is up to 25%.
Origin and pathophysiology
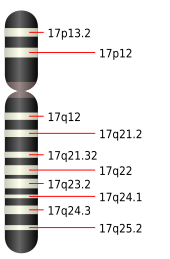
The cause of the development of Miller-Dieker syndrome is a chromosome aberration with microdeletions in the terminal short arm of chromosome 17 in the gene locus p13.3. This leads to a migration disorder of the nerve cells of the cerebral cortex in the embryonic brain development . The steps in brain development that take place from the 22nd week of embryonic development, during which the cerebral furrows form, are partially or completely absent. The fact that Miller-Dieker syndrome is a contiguous gene syndrome , in which several genes located close together can be affected, can lead to further genetic malformations, depending on the severity of the defect.
Diagnosis
The diagnosis can be made by means of cytogenetic testing of amniotic fluid obtained during amniocentesis . From the 26th week of pregnancy , the diagnosis can be made by sonography . In addition to microcephaly and agyria , enlargements of the cerebral ventricles , an abnormal skull shape, hydramnios , changes in the auricle and nose as well as general growth retardation can be visible. Depending on the extent of the chromosome damage, possible heart defects, kidney or gastrointestinal malformations as well as cryptorchidism can be shown. The extent of the brain damage can be determined prenatally either by a magnetic resonance tomography examination or by vaginal sonography. After birth, the diagnosis can be made by genetic testing using fluorescence in situ hybridization or DNA microarray . Magnetic resonance imaging can show the extent of lissencephaly.
clinic
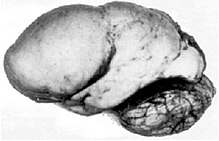
Children born with Miller-Dieker syndrome have severe intellectual disabilities. Pronounced muscular hypotension and a pronounced tendency to epileptic seizures are typical . The children often have massive respiratory and swallowing problems. The appearance is characterized by microcephaly with a high forehead and protruding occiput. In the area of the temples, there are indentations on both sides. The face has a narrow upper lip with a noticeably long philtrum . There is auricular dysplasia and a broad nasal root. In the brain, grooves and folds that have been lifted up to the full extent of lissencephaly can be found. The cerebral cortex is thickened and there are enlarged cerebral ventricles. It can be a corpus callosum are present or hypoplasia. In addition, depending on the extent of the genes involved, a microgenius , heart defects such as B. Fallot tetralogy , kidney malformations, malformations of the gastrointestinal tract, cryptorchidism , corneal opacities as well as campto- and clinodactylia occur.
therapy
A causal therapy for Miller-Dieker syndrome is not possible. If the parents have a balanced dislocation on chromosome 17, genetic counseling should be given. A new pregnancy can be done through artificial insemination with pre- implantation diagnostics . If the disease develops through a new mutation, the risk of a recurrence of Miller-Dieker syndrome is not increased. The therapy of affected children is symptomatic and includes the treatment of epilepsy , prophylaxis of pneumonia and other respiratory diseases as well as feeding by gastric tube .
forecast
Most of those affected die in early childhood from the consequences of nutritional problems , from aspiration-related respiratory diseases and general infections or from the consequences of constant epileptic seizures. Most children die by the age of two, with a few reaching the age of ten. The oldest known case, up to 2015, was 17 years old. There is currently a young man who was born in 1992.
Web links
- Miller-Dieker syndrome in the Genetics Home Reference of the United States National Library of Medicine
- Miller-Dieker Syndrome in the Online Mendelian Inheritance in Man
- Miller-Dieker Syndrome in the Diseases Database
- Miller-Diekes syndrome in the MeSH database of the United States National Library of Medicine
Individual evidence
- ↑ JQ Miller: LISSENCEPHALY IN 2 SIBLINGS. In: Neurology. Volume 13, October 1963, pp. 841-850, ISSN 0028-3878 . PMID 14066999 .
- ↑ H. Dieker, RH Edwards, G. ZuRhein and others: The lissencephaly syndrome. In: Birth defects Orig Art. Ser V, 1969 (2), pp. 53-64.
- ↑ Miller-Dieker Syndrome on Orphanet
- ↑ a b c d e f g h i William B. Dobyns, Soma Das: LIS1-Associated Lissencephaly / Subcortical Band Heterotopia . GeneReviews March 3, 2009
- ↑ a b c d e f g h Michael Entezami, Matthias Albig, Adam Gasiorek-Wiens, Rolf Becker: Sonographic malformation diagnostics: teaching atlas of fetal ultrasound examination. Georg Thieme Verlag, 2002, p. 248
- ^ WB Dobyns: Developmental aspects of lissencephaly and the lissencephaly syndromes. In: Birth defects original article series. Volume 23, Number 1, 1987, pp. 225-241, ISSN 0547-6844 . PMID 3472611 .
- ↑ Birgit Ertl-Wagner: Pediatric Neuroradiology. Springer 2007 p. 25
- ^ A b Gerhard Jorch : Fetoneonatal Neurology: Diseases of the nervous system from the 20th week of gestation to the 20th month of life. Georg Thieme Verlag, 2013, p. 206
- ^ A b Jürgen Kunze: Wiedemann's Atlas of Clinical Syndromes: Phenomenology, Etiology, Differential Diagnosis. Schattauer Verlag, 2010, p. 88