Motretinide
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
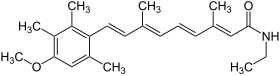
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Motretinide | ||||||||||||||||||
| other names |
all- trans - N -ethyl-9- (4-methoxy-2,3,6-trimethylphenyl) -3,7-dimethyl-2,4,6,8-nonatetraenamide ( IUPAC ) |
||||||||||||||||||
| Molecular formula | C 23 H 31 NO 2 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 353.51 g · mol -1 | ||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Motretinide [trade name: Tasmaderm ® (CH)] is a retinoid second generation (monoaromatic retinoids).
operation area
Motretinide is used as a pharmaceutical active ingredient and is used topically as an effective component of creams and solutions in the treatment of acne and other hyperkeratotic skin diseases . The drug is only approved in Switzerland .
effect
Motretinide 0.1% was found to be 0.05% more effective against inflammatory lesions than tretinoin , but not against comedolysis . Overall, it is rated as less effective. The formulation used has good skin tolerance.
Side effects
In the first few weeks of therapy, there may be temporary irritation of the skin and, in very rare cases, deterioration of the skin condition.
Interactions
Other external skin preparations should only be used concurrently with motretinide preparations after medical advice. The combined use of several topical acne medicines can cause dryness and irritation of the skin.
pregnancy and breast feeding period
Animal studies have shown undesirable effects on the fetus after systemic application of motretinide . There are no controlled human studies. Systemic uptake with topical application of the active ingredient is estimated at a maximum of 1%. Since the safety of topical application during pregnancy has not been systematically investigated, preparations containing motretinide should not be administered during pregnancy or if the patient wishes to have children. In addition, it is not known whether motretinide is excreted in breast milk. Use during breastfeeding is not recommended.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Zouboulis CC .: Modern Acne Therapy In: Akt Dermatol 2003; 29: 49-57. Georg Thieme Publishing House. Full text (PDF; 116 kB).
- ↑ Orfanos CE, Garbe C: 16.3.1 Effect on follicular hyperkeratosis In: Therapy of Skin Diseases , p. 383; 2nd edition, Springer 2002. ISBN 3-540-41366-9 .