Mukaiyama hydration
The Mukaiyama hydration is an organic chemical reaction catalyzed by the Japanese chemist Teruaki Mukaiyama was discovered (1927-2018).
Overview reaction
In Mukaiyama hydration, one equivalent of water is formally added to one alkene . The oxygen source for the resulting alcohol group is used atmospheric oxygen , hydrogen is from phenylsilane provided. This creates the Markovnikov product. Cobalt (II) acetylacetonate [Co (acac) 2 ] serves as the catalyst for this reaction :
mechanism
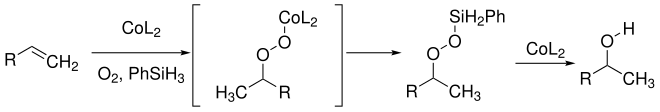
The mechanism of Mukaiyama hydration is not fully understood. Mukaiyama published the first mechanism in 1989. He assumes that alkene, cobalt catalyst (CoL 2 ), oxygen and phenylsilane (PhSiH 3 ) as a hydrogen source form a cobalt-peroxide adduct. A transmetalation creates a silyl peroxide. Under catalysis of the cobalt compound, the oxygen-oxygen bond is split and the alcohol is formed:
variants
In addition to the conversion of alkenes, there are also protocols for the hydration of α, β-unsaturated carbonyl compounds . Both the Mukaiyama and Magnus variants use a manganese- based catalyst in which the acetylacetonate is exchanged for 2,2,6,6-tetramethyl-3,5-heptanedione (dipivaloylmethane, dmp). While Mukaiyama's work is mainly concerned with the conversion of α, β-unsaturated benzyl esters , Magnus also succeeds in hydrating cyclic α, β-unsaturated keto compounds:
application
Mukaiyama hydration is used in the total synthesis of sesquiterpene (+) -Omphadiol. In the first step of the synthesis (R) - carvone is hydrated. In this case acts iso -propanol ( i -PrOH) as the solvent.
criticism
From the perspective of atomic economy, Mukaiyama hydration is one of the less efficient reactions, since, in addition to the target molecule (alcohol), considerable amounts of waste are generated in at least stoichiometric proportions. Therefore the application is limited to the laboratory scale.
Individual evidence
- ↑ Shigeru Isayama, Teruaki Mukaiyama: A New Method for Preparation of Alcohols from Olefins with Molecular Oxygen and Phenylsilane by the Use of Bis (acetylacetonato) cobalt (II) . In: Chemistry Letters . tape 18 , no. 6 , June 1989, pp. 1071-1074 , doi : 10.1246 / cl.1989.1071 .
- ↑ Satoshi Inoki, Koji Kato, Shigeru Isayama, Teruaki Mukaiyama: A New and Facile Method for the Direct Preparation of α-Hydroxycarboxylic Acid Esters from α, β- Unsaturated Carboxylic Acid Esters with Molecular Oxygen and Phenylsilane Catalyzed by Bis (dipivaloylmethanato) manganese ( II) Complex . In: Chemistry Letters . tape 19 , no. 10 , October 1, 1990, p. 1869–1872 , doi : 10.1246 / cl.1990.1869 .
- ↑ Philip Magnus, Andrew H Payne, Michael J Waring, David A Scott, Vince Lynch: Conversion of α, β- unsaturated ketones into α-hydroxy ketones using an MnIII catalyst, phenylsilane and dioxygen: acceleration of conjugate hydride reduction by dioxygen . In: Tetrahedron Letters . tape 41 , no. 50 , December 9, 2000, pp. 9725-9730 , doi : 10.1016 / S0040-4039 (00) 01727-5 ( sciencedirect.com [accessed January 12, 2020]).
- ↑ Gang Liu, Daniel Romo: Total Synthesis of (+) - Omphadiol . In: Angewandte Chemie International Edition . tape 50 , no. 33 , 2011, p. 7537-7540 , doi : 10.1002 / anie.201102289 .