Benzyl ester
Benzyl esters are chemical compounds that belong to the group of carboxylic acid esters . They are created by esterifying a carboxylic acid with benzyl alcohol (PhCH 2 OH). They have the characteristic functional ester group . In a broader sense, the benzyl esters also include carbonic acid esters and esters derived from sulfonic acids and benzyl alcohol. Similarly, benzyl esters in the broader sense can also form from nitric acid , sulfinic acids , phosphonic acids or phosphinic acids on the one hand and benzyl alcohol on the other.
Occurrence
Benzyl formate occurs naturally in various plants and mushrooms (for example potato rose , coffee, vanilla).
Manufacturing
Benzyl esters arise z. B. from a carboxylic acid and benzyl alcohol with acid-catalyzed dehydration . Benzyl esters can also be obtained from carboxylic acid chlorides and benzyl alcohol by the Schotten-Baumann method . Benzyl esters can also be synthesized from carboxylic acid anhydrides and benzyl alcohol . The nucleophilic substitution of benzyl halides (e.g. benzyl bromide 2 ) with alkali salts of carboxylic acids (e.g. benzoic acid sodium salt 1 ) leads to the benzyl ester 3 of benzoic acid:
When using phase transfer catalysts, it is possible to work under milder conditions and with higher yields.
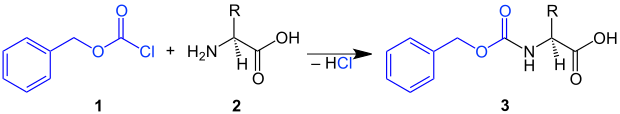
Use as a protecting group
In synthetic organic chemistry, and especially in peptide synthesis , the benzyloxycarbonyl group is introduced with benzyl chloroformate in the presence of a weak base . The Cbz group can be for protecting an amino group in a molecule easy to introduce, by the amine (z. B. the amino acid 2 ) with benzyloxycarbonyl chloride 1 in the presence of a weak base is reacted. This creates a Cbz -protected amino acid 3 :
The protected amine (e.g. in 3 ) can be deprotected again by catalytic hydrogenation with hydrogenolytic cleavage of the benzyl-oxygen bond with subsequent decarboxylation of the unstable carbamic acid thus formed or treatment with acids.
Individual evidence
- ↑ George A. Burdock: Fenaroli's Handbook of Flavor Ingredients, Fifth Edition: . CRC Press, 2004, ISBN 978-1-4200-3787-6 , pp. 1927 ( limited preview in Google Book search).
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig 1985, ISBN 3-342-00280-8 , p. 402.
- ^ Klaus Schwetlick et al: Organikum . 24th edition. Wiley-VCH, Weinheim 2015, ISBN 978-3-527-33968-6 .
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, p. 661, ISBN 3-342-00280-8 .