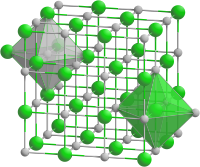
Sodium chloride structure
The crystal structure of sodium chloride (common salt, halite ) typical of many salts is the prototype for the sodium chloride structure (common salt structure ) or the sodium chloride type. Examples of representatives of this structure type are sodium chloride itself, lithium chloride (LiCl) and potassium chloride (KCl, Sylvin ), but also most alkaline earth oxides such as MgO . Exceptions that do not correspond to the sodium chloride type include cesium halides (with the exception of cesium fluoride ) and beryllium oxide (BeO).
If one takes the three spatial axes of a cube , which corresponds to the unit cell , then sodium and chloride ions are arranged alternately along the x, y and z axes. Each sodium ion is thus octahedral surrounded by six chloride ions and, conversely, each chloride ion is octahedral surrounded by six sodium ions in the form of an octahedron .
In general, a crystal structure is clearly described by a translation lattice and a base. Here, the forming face centered cubic lattice (fcc), the dot grid with the canonical unit vectors ( , , ) as the grating vectors and the lattice parameters. Each grid point is occupied by the following base:
- Na + bei , i.e. on the grid points of the fcc grid
- Cl - at , i.e. in the center of the fcc unit cell with edge length
In the case of other substances, the only difference is the type of ions at the corresponding lattice locations, with different ion radii also occurring. The ratio of these ionic radii is an important criterion for the formation of a sodium chloride type. You can see the sodium chloride structure as a cubic close packing (Engl. Closest cubic packed (ccp)) of the larger anions view in which the smaller cations the octahedral interstices occupy.
The lattice parameter of the sodium chloride unit cell is approximately 0.564 nm (5.64 Å ) at room temperature . NaCl crystals that have grown under microgravity in the ISS have the same crystal structure with identical lattice parameters.
Web links
Individual evidence
- ^ Pietro Fontana, Jürg Schefer, Donald Pettit: Characterization of sodium chloride crystals grown in microgravity . In: Journal of Crystal Growth . tape 324 , 2011, pp. 207–211 , doi : 10.1016 / j.jcrysgro.2011.04.001 .
literature
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 91st – 100th, improved and greatly expanded edition. Walter de Gruyter, Berlin 1985, ISBN 3-11-007511-3 .
- Pietro Fontana, The variety of salt crystals. Solothurn 2013, ISBN 978-3-033-04031-1