Neopentyl glycine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
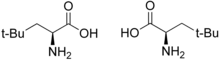
|
|||||||||||||||||||
| L -neopentylglycine (left) or D -neopentylglycine (right) | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Neopentyl glycine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 7 15 36 NO 2 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 145.20 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Neopentylglycine (abbreviated as Neo ) is a non-proteinogenic α- amino acid and can be produced using a modified Strecker reaction according to Patel & Worsley et al. getting produced.
The L - isomer can be isolated in good yields (approx. 75%) with an ee of 99.9% via an enzymatic reduction of the analogous 2-oxo acid ( 2-oxo-3,3-dimethylpentylic acid ) with LeuDH . The research group reports from a 30 kg approach.
The L - isomer is highly lipophilic and due to the space - filling structure this amino acid can e.g. B. be used for conformation studies of proteins .
According to the Sigma-Aldrich safety data sheet, the compound has not yet been fully analyzed for its toxicological and (bio) chemical properties.
Individual evidence
- ↑ a b c d data sheet L-α-Neopentylglycine from Sigma-Aldrich , accessed on May 22, 2017 ( PDF ).
- ↑ a b J. L. Fauchère, C. Petermann: Synthesis of gamma-methyl-L-leucine (neopentylglycine, Neo) and derivatives suitable for peptide synthesis. In: International journal of peptide and protein research. Volume 18, Number 3, September 1981, pp. 249-255, PMID 7341518 .
- ↑ G. Krix, S. Bommarius, Journal of Biotechnology 1997 , 53, pp. 29-39