Niobium electrolytic capacitor
A niobium electrolytic capacitor is a capacitor whose anode electrode is made of niobium or niobium (II) oxide , on which a uniform, electrically insulating layer of niobium pentoxide as a dielectric is generated by anodic oxidation , also known as formation . A solid electrolyte forms the cathode of the capacitor. Niobium electrolytic capacitors are polarized capacitors that may only be operated with direct voltage . The anode is the positive pole. Incorrect polarity, too high a voltage or ripple current overload lead to a short circuit and the destruction of the capacitors.
Construction, construction
Niobium electrolytic capacitors, like almost all capacitors in electronics , are basically plate capacitors, the capacity of which is greater, the larger the electrode area A and the relative permittivity ε and the closer the electrodes are to one another (d).
The basic material of the niobium electrolytic capacitor is an anode made of fine-grained sintered niobium or niobium oxide powder. A large number of pores remain in the sintered niobium block, which run through the entire sintered block and thus create a very large surface area of the anode (many times the surface of a smooth block). This increase in surface area is an important factor that contributes to the relatively high specific capacitance of niobium electrolytic capacitors compared to other capacitor families.
The surface of the anode is then "anodically oxidized" or formed in an electrolysis bath. By applying a current source with the correct polarity on the niobium surface, a uniform and electrically insulating layer of niobium pentoxide (Nb 2 O 5 ) is formed. This oxide layer is the dielectric of the capacitor.
The dielectric strength of niobium pentoxide is very high at around 455 V / µm. Since any desired dielectric strength can be achieved through the formation, the thickness of the oxide layer varies with the nominal voltage of the later capacitor. A 10 V niobium electrolytic capacitor therefore has a dielectric with a layer thickness of only about 0.022 µm. This extremely thin dielectric is the second important factor that contributes to the relatively high specific capacitance of niobium electrolytic capacitors compared to other capacitor families.
A conductive material, the electrolyte, which in niobium electrolytic capacitors can consist of manganese dioxide or a conductive polymer , forms the cathode of the capacitor. It adapts completely to the surface structure of the anode and the dielectric on it. The electrolyte must then be contacted with the external cathode connection by suitable means. At the end, the entire construction is wrapped in plastic to protect it against environmental influences.
| Anode material | dielectric | dielectricity number |
Dielectric strength in V / µm |
|---|---|---|---|
| aluminum | Aluminum oxide, Al 2 O 3 | 9.6 | 700 |
| Tantalum | Tantalum pentoxide, Ta 2 O 5 | 26th | 625 |
| niobium | Niobium pentoxide, Nb 2 O 5 | 42 | 455 |
Compared to tantalum, niobium has a higher dielectric constant, but a lower dielectric strength of the dielectric. Both parameters mean that the volume efficiency of niobium electrolytic capacitors is quite comparable to that of tantalum electrolytic capacitors .
Niobium electrolytic capacitors are in direct competition with tantalum electrolytic capacitors, provided that both use a manganese dioxide electrolyte. When using polymer electrolytes, with which extremely low internal losses can be achieved, niobium electrolytic capacitors also compete with MLCC ceramic capacitors , plastic film capacitors , tantalum and aluminum electrolytic capacitors with polymer electrolytes.
history
Like tantalum and aluminum, niobium is a so-called valve metal that forms an electrically blocking insulating layer during anodic oxidation, which can be used as the dielectric of a capacitor. The principle had been known since the beginning of the 20th century, the technical difficulties of the material with its high melting point of 2744 ° C prevented its implementation at the time.
It was the availability of the base metal at the end of the 1960s that led to the development of niobium electrolytic capacitors in what was then the Soviet Union, where they took the place of the military tantalum electrolytes with sintered anodes and manganese dioxide electrolytes in the west.
With the collapse of the Iron Curtain, this know-how also became public in the West. Since niobium is available as a raw material much more frequently than tantalum and is also cheaper, the major manufacturers became interested in this technology at the end of the 1990s.
The biggest problem with niobium electrolytic capacitors was getting the residual current under control. Because unlike tantalum or aluminum, niobium forms different oxide layers. NbO and NbO 2 are suboxides of niobium, which are metallically conductive and semiconducting, which can lead to a higher leakage current or even to defects in the capacitor. In addition, the solubility of oxygen in niobium is higher than in tantalum, which in a niobium / niobium oxide layer system favors the formation of undesirable suboxides, especially at higher temperatures. For these reasons, the dielectric layer of niobium electrolytic capacitors must be stabilized, which requires precise process control.
The difficulties in getting the residual current of niobium electrolytic capacitors under control led to a new type of niobium oxide electrolytic capacitors, called "oxicaps", which use niobium [II] oxide (NbO) as the anode material. The suboxide NbO is a ceramic substance and has a metallic conductivity that is not as good as the pure metal niobium, but is low enough for use as an anode material.
The main objective of the developments in recent years is to reduce internal ohmic losses, which are summarized in the so-called "ESR", the equivalent series resistance in the series equivalent circuit diagram.
As with tantalum chip electrolytic capacitors, two approaches are taken for this purpose. On the one hand there is the multiple anode technology, in which several anode blocks are connected in parallel in one housing. This achieves ESR values of 20 to 30 mΩ. On the other hand, by using a polymer electrolyte, ESR values in the single-digit mΩ range can be achieved. As a result, niobium electrolytic capacitors today achieve values that are in direct competition with ceramic multilayer film capacitors (MLCC).
Design
Niobium electrolytic capacitors are only manufactured as surface mount SMD chip capacitors. They are available in two different versions:
- Niobium SMD electrolytic capacitors with sintered anode and solid electrolyte manganese dioxide
- Niobium oxide SMD electrolytic capacitors with sintered anode and solid electrolyte manganese dioxide
particularities
The characteristics of electrolytic capacitors have some special features that distinguish them from other types of capacitors. For special information on capacity, capacity tolerance, dielectric strength, residual current, current carrying capacity, recharging effect (dielectric absorption), impedance or impedance behavior, see electrolytic capacitor
Series equivalent circuit diagram, circuit diagram and marking
polarity
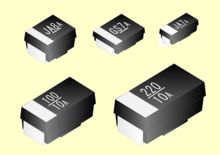
The positive connection is identified by a bar (colored line) on the housing, as shown in the designs shown in the middle in cuboid, yellow and black plastic housings. This bar can easily be mistaken for a minus sign, which in turn leads to errors, because with the SMD design of aluminum electrolytic capacitors (V-Chip) in the typical cylindrical aluminum housing, the bar marks the negative connection. In the illustration this is the black mark on the two housings on the far left.
Advantages and disadvantages
- advantages
- similarly high storage density as tantalum electrolytic capacitors
- very reliable, no useful life / service life limited by evaporation
- good low temperature behavior.
- lighter than tantalum electrolytic capacitors
- comparable with ceramic MLCC capacitors due to the low ESR
- less flammable than tantalum electrolytic capacitors in the event of a short circuit
- cheaper than tantalum electrolytic capacitors
- disadvantage
- more expensive than alumina electrolytic capacitor
- The capacity range offered is still relatively small, the nominal voltage range ends at 25 V, the max. permissible operating temperature is currently 105 ° C.
- higher residual current than with tantalum electrolytic capacitors
- So far the number of manufacturers is small.
Applications
- Secondary filter capacitor in miniaturized SMPS-AC-DC converters in base stations, networks, motherboards, mobile phones, laptops
swell
- Ch. Schnitter, A. Michaelis, U. Merker, HC Starck: New Niobium Based Materials for Solid Electrolyte Capacitors . Carts 2002.
- T. Zednicek, S. Sita, C. McCracken, WA Millman, J. Gill, AVX, “Niobium Oxide Technology Roadmap”, CARTS 2002 [1] .
- T. Zednicek, B. Vrana, WA Millman, J. Gill, Ch. Reynolds, AVX, Tantalum and Niobium Technology Roadmap [2]
- Y. Pozdeev-Freeman, P. Maden: Solid-Electrolyte Niobium Capacitors Exhibit Similar Performance to Tantalum . February 1, 2002. URL: [3]
- Ch. Schnitter, Starck: The taming of niobium . Starck company publication, 2002.
- T. Zednicek, S. Zednicek, S. Sita, C. McCracken, WA Millman: Low ESR and Low Profile Technology on Niobium Oxide . AVX 2003.
- WA Serjak: Tantalum-Niobium International Study Center . Brussels, 2004.
Web links
- AVX, product overview niobium oxide electrolytic capacitors [4] and [5]
- Kemet, research report (PDF file; 53 kB)
- NEC Tokin, FAQ
- Vishay, press release, [6]