N -nitrosamides
| Basic body with functional group
marked in blue |
N -nitrosamide derivatives with functional groups
marked in blue |
|
N -nitrosamides |
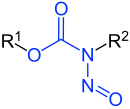 |
| R 1 to R 3 are hydrogen atoms or organic radicals | |
Nitrosamides , also N -nitrosamides, belong, like the N -nitrosamines, to the group of nitroso compounds . In the case of nitrosamides, it is chemically reactive and metabolically unstable nitrosated ureas, guanidines or carbamates. They are generally carcinogens. In the discussion about impurities resulting from the environment , the N -nitrosamides, in contrast to the N -nitrosamines, do not play a role.
Use
Various chloroethylnitrosoureas, such as N, N′-bis (2-chloroethyl) -nitrosourea ( BCNU ), have achieved medical benefits in the fight against malignant tumors . Assumptions about the effectiveness against cancer cells are based on the alkylatability of guanine - cytosine centers in the sequences of the genome , especially the oncogenes .
synthesis
N -Nitrosamide can be prepared starting from the reaction of N -monosubstituierten acid amides and the, in the presence of strong acids from the nitrous acid arising, nitrosyl cation produced, here exemplified by the N -Methylacetamids ( 1 ). A nucleophilic attack on the nitrosyl cation occurs from the acid amide. After a proton has been split off, the resulting cation forms an N -nitrosamide ( 2 ):
toxicity
The genetic damaging effect of the N -nitroso compounds can be traced back to the formation of reactive electrophilic particles in the metabolism. The spontaneous decomposition of N -nitrosoureas in the aqueous medium of metabolism, here using the example of 1-methylnitrosourea ( 3 ), diazonium or carbenium ions are formed . The decomposition takes place in isocyanic acid and methyldiazohydroxid. The rearrangement to the diazonium ion and the subsequent elimination of nitrogen produces a carbenium ion ( 4 ), which can alkylate nucleophilic intersections of the DNA.
The decomposition of N -nitrosoureas with a higher degree of substitution can take place in the organism . An alternative way of creating diazonium and carbenium ions is through the enzymatic reaction of nitrosamines.
Typical side effects in the context of medical cancer treatment using N -nitrosoureas are the impairment of the bone marrow (damage to the stem cell compartment), the lymphatic tissue and the gastrointestinal tract.
Individual evidence
- ↑ a b c Hans Marquardt, Siegfried G. Schäfer (Ed.): Textbook of Toxicology. 2nd edition, Wissenschaftliche Verlagsgesellschaft mbH Stuttgart, 2004, ISBN 3-8047-1777-2 , p. 747.
- ↑ a b Hans Marquardt, Siegfried G. Schäfer (Ed.): Textbook of Toxicology. 2nd edition, Wissenschaftliche Verlagsgesellschaft mbH Stuttgart, 2004, ISBN 3-8047-1777-2 , pp. 752-753.
- ^ Adalbert Wollrab: Organic chemistry. An introduction for teacher training and minor students. 4th edition, Springer-Verlag Berlin Heidelberg 2014, ISBN 978-3-642-45144-7 , p. 898.
- ↑ Heinz GO Becker, Rainer Beckert, Werner Berger, Günter Domschke, Egon Fanghänel, Mechthild Fischer, Frithjof Gentz, Karl Gewald, Reiner Gluch, Wolf D. Habicher, Hans-Joachim Knölker, Roland Mayer, Peter Meth, Klaus Müller, Dietrich Pavel , Hermann Schmidt, Karl Schollberg, Klaus Schwetlick, Erika Seiler, Günter Zeppenfeld: Organikum. 24th edition, Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim 2015, ISBN 978-3-527-33968-6 , p. 648.
- ↑ a b c d e f Hans Marquardt, Siegfried G. Schäfer (Ed.): Textbook of Toxicology. 2nd edition, Wissenschaftliche Verlagsgesellschaft mbH Stuttgart, 2004, ISBN 3-8047-1777-2 , pp. 753-758.




