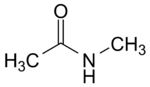
N -methylacetamide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | N -methylacetamide | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 3 H 7 NO | |||||||||||||||
| Brief description |
colorless solid with a faint odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 73.09 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
0.94 g cm −3 |
|||||||||||||||
| Melting point |
27-30.6 ° C |
|||||||||||||||
| boiling point |
206-208 ° C |
|||||||||||||||
| Vapor pressure |
1.1 hPa (50 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.433 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Authorization procedure under REACH |
of particular concern : toxic for reproduction ( CMR ) |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
N -Methylacetamide is a chemical compound from the group of carboxamides . The compound is one of the substances of very high concern of the European Chemicals Agency (ECHA).
Extraction and presentation
N -Methylacetamide can be obtained by reacting hot acetic acid or acetic anhydride with methylamine . It is also possible to produce it by reacting N , N '-dimethylurea with acetic acid or by reacting acetone oxime with sulfuric acid .
properties
N -Methylacetamide is a flammable, hardly inflammable, hygroscopic , crystalline, colorless solid with a faint odor, which is soluble in water. Several isomeric forms are known. In solution it is 97–100% as a ( Z ) isomer with a polymeric structure. The compound has a high dielectric constant of 191.3 at 32 ° C.
use
N -Methylacetamide is used as an intermediate in the manufacture of agrochemicals and as a solvent in electrochemistry.
Individual evidence
- ↑ a b c d e f g h i j k Entry on N-methylacetamide in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ a b J. F. Coetzee: Recommended Methods for Purification of Solvents and Tests for Impurities International Union of Pure and Applied Chemistry . Elsevier, 2013, ISBN 978-1-4831-3845-9 , pp. 50 ( limited preview in Google Book search).
- ↑ a b Data sheet N-Methylacetamide, 99% from AlfaAesar, accessed on April 17, 2017 ( PDF )(JavaScript required) .
- ↑ Data sheet N-Methylacetamide, ≥99% from Sigma-Aldrich , accessed on April 17, 2017 ( PDF ).
- ↑ Entry on N-methylacetamide in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on April 29, 2017. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Entry in the SVHC list of the European Chemicals Agency , accessed on April 21, 2020.
- ↑ a b Entry on N-methylacetamide in the Hazardous Substances Data Bank , accessed April 17, 2017.
- ↑ a b D.R. Buhler, DJ Reed: Nitrogen and Phosphorus Solvents . Elsevier, 2013, ISBN 978-1-4832-9020-1 , pp. 166 ( limited preview in Google Book search).
- ↑ Joachim Buddrus, Bernd Schmidt: Fundamentals of organic chemistry . Walter de Gruyter GmbH & Co KG, 2015, ISBN 978-3-11-033105-9 , p. 581 ( limited preview in Google Book search).
- ↑ Noemi G. Mirkin, Samuel Krimm: Conformers of cis-N-methylacetamide. In: Journal of Molecular Structure: THEOCHEM. 236, 1991, p. 97, doi : 10.1016 / 0166-1280 (91) 87010-J .
- ↑ Ab initio vibrational analysis of hydrogen-bonded trans- and cis-N-methylacetamide . In: Journal of the American Chemical Society . tape 113 , no. 26 , December 1, 1991, pp. 9742-9747 , doi : 10.1021 / ja00026a005 .
- ↑ K.-H. Hellwich: Basic stereochemistry terms . Springer-Verlag, 2013, ISBN 978-3-662-10051-6 , pp. 43 ( limited preview in Google Book search).
- ^ A. Covington: Physical Chemistry of Organic Solvent Systems . Springer Science & Business Media, 2012, ISBN 978-1-4684-1959-7 , pp. 247 ( limited preview in Google Book search).
