Carmustine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
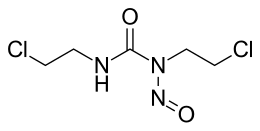
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Carmustine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 5 H 9 Cl 2 N 3 O 2 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 214,05 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
30 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Carmustine (also B is- C hlorethyl- N itroso- U rea, abbreviated to BCNU ) is a cytostatic effective drug , the specific in the treatment of severe, advanced cancers is used. Because of its strong side effects, carmustine has very limited uses. The German Society for Hematology and Medical Oncology (DGHO) judges carmustine to be irreplaceable for preparing patients for a stem cell transplant .
Chemical classification
Chemically, the substance belongs to the group of nitrosoureas , whose effect against cancer cells is based on the alkylation of nucleic acids (DNA, RNA) ( alkylating agents ).
Pricing policy
After the original manufacturer sold the approval of the carmustine preparation Carmubris to the Indian company Emcure Pharmaceuticals in 2013 and ceased sales in Germany in 2014, the new license holder's agent is imported for the German market via its sales company. The price of the drug multiplied. While 100 milligrams cost 35 euros in 2013, the price rose forty-fold by 2018 to 1,400 euros.
See also
Commercial preparations
Carmubris (A), Gliadel (D, A)
In July 2018, the first generic carmustine was approved in the EU for the treatment of brain tumors ( glioblastoma , brainstem glioma , medulloblastoma , astrocytoma and ependymoma ), brain metastases and second therapy for non-Hodgkin lymphoma and Hodgkin's disease .
Individual evidence
- ↑ a b c data sheet carmustine from Sigma-Aldrich , accessed on March 15, 2011 ( PDF ).
- ^ Entry on carmustine. In: Römpp Online . Georg Thieme Verlag, accessed on June 14, 2014.
- ↑ Only one manufacturer worldwide and now a dramatic increase in the price of cancer drugs , DGHO press release on January 30, 2015.
- ↑ Birgit Augustin: Sharp rise in the price of cancer drugs | Plus minus. June 27, 2018, archived from the original on June 27, 2018 ; accessed on June 27, 2018 (German).
- ↑ carmustine Obvius on the side of the European Medicines Agency. Retrieved December 9, 2018.