Orton rearrangement
The Orton rearrangement is a name reaction from the field of organic chemistry , which was first published in 1886 by the chemist G. Bender and in 1890 by the English chemist HE Armstrong (1848–1937) and in 1899 by the chemist KJP Orton (1872–1930) has been extensively researched with the following result. The Orton rearrangement describes the conversion of an N - chloro - acylanilide under the action of acid into an N- acyl- para- chloroanilide.
Overview
An N -chloro-acylanilide is converted into an N -acyl- para -chloroanilide under acid catalysis :

There is a rearrangement of the chloride group ( Cl ) from the anilide nitrogen atom to thereto in the para position located carbon atom of the benzene instead.
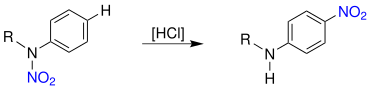
Under analogous reaction conditions (acid catalyzed) an N -nitro-alkyl aniline can be converted to form an N -alkyl para -nitroaniline:
The nitro group ( NO 2 ) is rearranged analogously to the chloride group.
Reaction mechanism
The following illustrations describe two alternative proposals for the reaction mechanism:

As an electrophile , the hydrogen atom of the hydrochloric acid molecule binds as a proton to the nitrogen atom of the N -chloro-acylanilide 1 . The chloride cation split off by the electrophilic substitution reacts with the chloride anion of the hydrochloric acid molecule to form chlorine. This is how the anilide 2 is created . A subsequent substitution reaction in the para position produces 4-chloroanilide 3 .

First, the hydrogen proton of the hydrochloric acid molecule binds to the nitrogen atom of the N -chloro-acylanilide 1 . The chloride group is split off as a chlorine radical. This chlorine radical is then bound to the benzene in the para position. A subsequent deprotonation by the chloride anion of the hydrochloric acid molecule in the para position produces 4-chloroanilide 2 .
Individual evidence
- ↑ G. Bender, Reports of the German Chemical Society , 1886 , Vol. 19, p. 2272.
- ^ HE Armstrong, J. Chem. Soc. , 1900 , vol. 77, p. 1047.
- ↑ FD Chattaway, KJP Orton, J. Chem. Soc. , 1899 , vol. 75, p. 1046.
- ↑ ICR Medina, JR Hanson, J. Chem. Res. , 2003 , p. 428.
- ↑ a b c d e f g h i j k Z. Wang (Ed.): Comprehensive Organic Name Reactions and Reagents, 3 Volume Set , John Wiley & Sons, Hoboken, New Jersey, 2009 , ISBN 978-0-471- 70450-8 , pp. 2093-2094.